Volume : 1 | Issue : 1
Review
Update on the management of pediatric thyroid cancer
Vera Zdravkovic
Division of Endocrinology, University Children’s Hospital, Belgrade
Received: March 19, 2018 | Published: April 10, 2018
Abstract
Objective: Although pediatric thyroid carcinomas in childhood are rare, incidence of papillary thyroid carcinoma (PTC) is increasing in in last decade. Medullary thyroid carcinomas (MTC) are usually component of MEN 2A and MEN 2B. Aim of this review is to provide an update on diagnosis and treatment of thyroid carcinoma in children.
Background: Pediatric thyroid carcinomas differ from the carcinoma in adults in pathophysiology, clinical presentation, and long-term outcomes. Initial presentation may be the lymph node or distant metastasis. MTCs are very aggressive and prophylactic thyroidectomy should be performed in first years of life.
Methods: New ATA recommendations and published reports of series of patients with PTC and MTC are reviewed and summarized.
Discussion: Pediatric endocrinologist should diagnose TC, refer to pediatric surgeon in timely manner and follow up those children after surgery. This review tends to give the practical approach and facilitate this process.
Conclusion: PTC and MTC are very aggressive and should be treated at initial phase. Early treatment of PTC and prophylactic thyroidectomy of MTC provides definitive cure.
Keywords: pediatric thyroid carcinoma, medullary thyroid carcinoma, children
Introduction
Thyroid carcinomas are rare in childhood; occurrence is 1% at the age 5–9 and 7% at 15-19 years.1 It is more common (2.5–6) in females after puberty. Papillary thyroid carcinoma (PTC) is the predominant variant at this age.2 Clinical presentation is different than in adults, initial presentation could be a palpable nodule (35–83%), lymph node metastasis (in up to 80%), but pulmonary metastasis are present in 16%.3 Medullary carcinoma could be sporadic or hereditary as a part of medullary endocrine neoplasia.4
Thyroid nodule
Thyroid nodules are also rare in childhood, usually are benign, but a unilateral cervical lymph node is always a bad prognostic sign.5 Once a thyroid nodule is identified, the overall risk of malignancy is higher for children (22%) than for adults (5–10%). The causes could be iodine deficiency or it could be a part of genetic syndromes like: Carney, DICER1, Werner, Beckwith–Wiedemann, familial paraganglyosis, Li–Fraumeni, McCune–Albright and Peutz–Jeghers. Cancer survivors should be monitored annually, especially those who had Hodgkin lymphoma, ALL or brain tumors. The irradiation of the neck and thyroid gland with 20-29 Gray represents high risk, which increases 10 years after radiotherapy.6
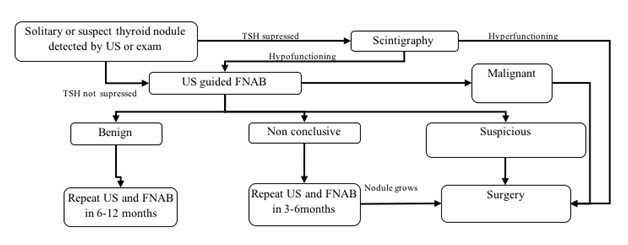
Thyroid nodule evaluation is presented in Figure 1. Thyroid function tests, neck ultrasound and fine needle aspiration biopsy (FNAB) are diagnostic steps as it is shown. Surgery should be performed depending on cytological findings. Benign nodule should be followed by the ultrasound every 1–2 years and FNAB performed if nodule grows or change its characteristics. Lobectomy is suggested in case of compression or due to esthetic reasons. Surgery is necessary if nodule is solid, bigger than 4 cm or in case of malignant transformation.7-9
Papillary carcinoma
High prevalence of gene rearrangements and a lower frequency of point mutations in the proto-oncogenes occur in PTC. Some of oncogenes are (RAS, RET, TRK) and tumor suppressive genes (p53, Rb, p16/CDKN2, p21). RET/PTC3 rearrangements are associated with more aggressive tumors and RET/PTC1 with classical PTC.10 Irradiation of head and neck and exposure to radionuclides (Chernobyl disaster) places a great risk for cancer development.11-12 It seems that younger children (<10 years of age) are at greater risk and have more severe disease with a risk of recurrence. ATA pediatric committee recommended that children with PTC should be divided into “prepubertal” and “pubertal/postpubertal” groups to search for the potential influence of pubertal development on the incidence and behavior of PTC within the pediatric population.12
Presentation of PTC could be various. Hard, non-painful and non-functional nodule in thyroid gland is very suspicious for carcinoma. Diffuse infiltration might lead to node enlargement and hard diffuse goiter could be the initial sign. In some patients lymph nodes may be present in the lower part of the neck without accompanied thyroid nodule.3,13 Solid nodule or micro calcifications in thyroid gland detected by the ultrasound or cold nodule on scintigraphy warrants urgent FNAB. In patients with large thyroid masses, CT or MRI should be performed trying to detect metastatic disease to deep tissue regions, such as the superior mediastinum, the retropharyngeal, parapharyngeal, and subclavicular spaces.13 PTC in childhood could be very aggressive. Cervical lymphadenopathy, hoarseness (infiltration of n. laryngeal reccurens) and pulmonary metastasis are warning signs of local and aggressive spreading.11 If the diagnosis is made when the cancer is in the early stage, the prognosis is good.6,12
Guidelines for preoperative staging and follow up are summarized in Table 1. Surgical approach depends on the preoperative staging. Total thyroidectomy with dissection of affected lymph nodes should be performed in all patients.13-14 If metastases are detected, more radical approach is needed. Bilateral dissection places patient at substantial risk of hypocalcemia and perioperative PTH measurement may help to determine the need for early calcium supplementation.15
|
Recommendation |
|---|---|
Pre-operative staging |
To identify patients who will need central dissection and RI therapy |
Surgery
|
Total thyroidectomy |
Lymph node dissection |
In patients with preoperative evidence of central and/or lateral |
Radioactive iodine |
The goal of 131I therapy is to decrease the risks of thyroid |
Follow up |
US of neck and Tg measurement. No routine whole body scan |
Therapy goal |
RI repeated therapy only in those with progressive disease. Substantial number of patients with pulmonary metastasis would not get into remission but will have a stable form of disease |
Table 1 Recommendation for diagnosis and follow up of children with PTC
Postoperative staging using TNM (tumor, nodule, and metastasis) nomenclature is suggested. This classification enables easier patient’s low to high risk stratification. Guidelines for PTC follow up are given in Table 2.13 Therapy with L-thyroxine should aim to suppress TSH to the lowest possible level according to risk level. Ultrasound and thyroglobulin (Tg) measurement are the best option to screen for the cancer recurrence.15-17
ATA pediatric risk level |
definition |
TSH level |
FU |
Remission FU |
|---|---|---|---|---|
Low risk |
Only thyroid gland (N0/Nx)/microscopic metastasis |
0,5–1,0 |
Tg |
US 6 months after surgery, then annually next 5 years |
Intermediate risk |
N1a or N1b |
0,1–0,5 |
Tg and 123I scan |
US 6 months after surgery every 6–12 months next 5 years. |
High risk |
(N1b) or T4 with or without distant metastasis |
< 0,1 |
TSH-stimulated Tg and diagnostic 123I scan |
Same as above |
Table 2 Risk stratification and follow up of PTC patients in childhood.
Medular carcinoma
Medular thyroid carcinoma (MTC) in childhood usually presents as a part of multiple endocrine neoplasia (MEN2A and MEN2B), including pheochromocytoma and parathyroid hyperplasia. The inheritance pattern of hereditary form of MTC is autosomal dominant with variable expression and age-dependent penetrance. MTC is the first symptom of MEN2A and MEN2B. It occurs within the first two decades of life in MEN2A and much earlier in MEN2B, sometimes presenting in infant age.18 Germ-line mutations in RET proto-oncogene are responsible for these syndromes and they are distinguished by specific mutations which differ among affected families. MEN2B could be hereditary (25%) or de novo mutation (75%). Some of these patients with de novo RET codon M918T should be screened for pheochromocytoma or thyroid nodule. They have characteristic phenotype (marfanoid habitus, elongated face, very bulging lips, multiple mucosal neuroma on tongue, lips and palpebral conjunctiva).19 It is important to seek for this phenotype, since younger children have lower calcitonin levels, smaller tumors and rare metastasis. Thyroidectomy at very young age, before 5 years, provides total cure.20
Children with hereditary MEN2A and MEN2B medullary carcinoma could have metastasis before 5 years of life and prophylactic thyroidectomy is necessary. Patients with RET codon M918T (MEN2B) mutation have very aggressive form and thyroidectomy should be performed in the first year of life.21-22 There is risk of permanent hypoparathyroidism since it is difficult to differentiate parathyroid gland at this age. Because of all these risks thyroidectomy should be performed in tertiary centers with experienced team (surgeon, anesthesiologist, pediatric endocrinologist).22
Guidelines for treatment and follow up of children with hereditary medullary thyroid carcinoma (MEN2A and MEN2B) are summarized in Table 3&4.18
MEN2A |
High risk |
Intermediate risk |
Team decision |
|---|---|---|---|
RET codon |
634 |
|
|
Age of carcinoma |
First year |
Less aggressive, after second year |
Family, surgeon, pediatric endocrinologist |
Recommendation |
From third year: palpation, US, calcitonin every 6 months |
After 5th year: examination, US, calcitonin every 6 months |
|
Thyroidectomy |
Before 5 years or age, earlier if calcitonin is elevated |
In adolescency or earlier if calcitonin is elevated. |
Table 3 Guidelines for treatment and follow up of patients with MEN2A
MEN2B |
Highest risk |
High risk |
Intermediate risk |
Team decision |
|---|---|---|---|---|
RET codon |
M918T |
A883F, V804MY806C, S904C, E805K, Q781R |
Family, surgeon, pediatric endocrinologist |
|
Thyroidectomy |
First year |
Before 5 years or earlier if calcitonin is elevated |
After 5 years or earlier if calcitonin is elevated. |
|
Central neck dissection |
Affected lymph nodes or high calcitonin |
Lymph or distant metastasis or calcitonin >40 pg/mL |
|
|
Table 4 Guidelines for treatment and follow up of patients with MEN2B
Calcitonin should be measured every 6 months and if it is high, the surgery should not be postponed. Normal reference range of calcitonin is bellow 30 pg/mL in the first year of life and 6 months of age and up to 10 pg/mL in the second year of life. Serum calcitonin values in children older than 3 years of age are indistinguishable from those observed in adults.23 Carcinoembryonic antigen (CEA) is not a specific biomarker for MTC and should not be used in the early diagnosis of MTC. Measurement of serum CEA levels is useful for evaluating disease progression in patients with clinically evident MTC and for monitoring patients after thyroidectomy.18
Children with MEN may develop pheochromocytoma very early, at 8 (ATA highest risk), 12 (ATA high risk) and 19 years of age (ATA moderate risk). Those patients should be followed by pediatric endocrinologist and screened for pheochromocytoma.17 Guidelines are given in Table 5.
|
Highest risk |
High risk |
Intermediate risk |
|---|---|---|---|
Age (years) |
11 |
11 |
16 |
Metanephrines and normetanephrines in serum or urine |
|||
Table 5 Screening for pheochromocytoma in children with MEN2A and 2B
Discussion
New ATA guidelines for thyroid carcinoma were published in 2015 and the aim of this review was to focus on the implementation these guidelines in the pediatric population.6,13 Before Chernobyl disaster, PTC was rare disease in childhood, but we are aware of the increasing incidence in the last decades. Siegel et al. reported that thyroid cancer incidence rates increased by 4.9% per year among US children and adolescents during 2001–2009.24 Although enlarged neck lymph node is usually sign of acute infection, any lymph node resistant to antibiotic treatment should not be ignored. The most frequent indication for thyroid imaging is a palpable mass in the neck or thyroid gland.5,8 Thyroid US is a first-line examination for visualizing the thyroid gland as it provides anatomic and perfusion information. Normal calcitonin levels would exclude the diagnosis of medullary carcinoma. FNA should be performed in thyroid nodes bigger than 2 cm. Recommendations regarding care for offspring of MEN parents are very clear. Since MTCs are very aggressive, prophylactic thyroidectomy should be performed in the first years of life, depending on the mutation.18 They should be followed by pediatric endocrinologists and advised about all possible risks in future life.
Conclusion
Thyroid cancer is rare disease in children and adolescents, papillary thyroid cancer being the most common type. Its clinical presentation could be thyroid nodule, lymph node or pulmonary metastasis. Total thyroidectomy should be performed in all patients. Medullary thyroid carcinoma is usually part of MEN2A and MEN2B. In patients with known mutation prophylactic thyroidectomy should be done in the first years of life.
References
- Dinauer C, Francis GL. Thyroid cancer in children. Endocrinol Metab Clin North Am. 2007;36(3):779‒806.
- Dinauer CA, Breuer C, Rivkees SA. Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol. 2008;20(1):59‒65.
- Dzodic R, Buta M, Markovic I, et al. Surgical management of well-differentiated thyroid carcinoma in children and adolescents: 33 years of experience of a single institution in Serbia. Endocr J. 2014;61(11):1079‒1086.
- Starenki D, Park JI. Pediatric Medullary Thyroid Carcinoma. J Pediatr Oncol. 2015;3(2):29‒37.
- Corrias A, Mussa A, Baronio F, et al. Diagnostic features of thyroid nodules in pediatrics. Arch Pediatr Adolesc Med. 2010;164(8):714‒719.
- Francis GL, Waguespack SG, Bauer AJ, et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2015;25(7):716–759.
- Kambalapalli M, Gupta A, Prasad UR, et al. Ultrasound characteristics of the thyroid in children and adolescents with goiter: a single center experience. Thyroid. 2015;25(2):176–182.
- The Canadian pediatric thyroid nodule study group. The Canadian pediatric nodule study: an evaluation of current management practices. J Pediatric Surg. 2008;43:826‒830.
- Guille JT, Opoku-Boateng A, Thibeault SL, et al. Evaluation and management of the pediatric thyroid nodule. Oncologist. 2015;20(1):19‒22.
- Prescott JD, Zeiger MA. The RET oncogene in papillary thyroid carcinoma.
- Cancer. 2015;121(13):2137‒2146.
- Waguespack SG, Francis G. Initial management and follow up of differentiated thyroid cancer in children. J Natl Compr Canc Netw. 2010;8(11):1289‒1300.
- Chan CM, Young J, Prager J, Travers S. Pediatric Thyroid Cancer. Adv Pediatr. 2017;64(1):171‒190.
- Francis GL, Waguespack SG, Bauer AJ, et al. American Thyroid Association Guidelines Task Force. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2015;25(7):716‒759.
- Verburg FA, Van Santen HM, Markus Luster. Pediatric papillary thyroid cancer: current management challenges. Onco Targets Ther. 2017;10:165–175.
- Chen Y, Masiakos PT, Gaz RD, et al. Pediatric thyroidectomy in a high volume thyroid surgery center: Risk factors for postoperative hypocalcemia. J Pediatr Surg. 2015;50(8):1316‒1319.
- Hoofnagle AN, Roth MY. Clinical review: improving the measurement of serum thyroglobulin with mass spectrometry. J Clin Endocrinol Metab. 2013;98(4):1343–1352.
- Padovani RP, Robenshtok E, Brokhin M, et al. Even without additional therapy, serum thyroglobulin concentrations often decline for years after total thyroidectomy and radioactive remnant ablation in patients with differentiated thyroid cancer. Thyroid. 2012;22(8):778–783.
- Wells SA, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroidcarcinoma. Thyroid. 2015;25(6):567‒610.
- Giri D, McKay V, Weber A, et al. Multiple endocrine neoplasia syndromes 1 and 2: manifestations and management in childhood and adolescence. Arch Dis Child. 2015;100(10):994‒999.
- Elisei R, Romei C, Renzini G, et al. The timing of total thyroidectomy in RET gene mutation carriers could be personalized and safely planned on the basis of serum calcitonin: 18 years experience at one single center. J Clin Endocrinol Metab. 2012;97:426–435.
- Pelizzo M, Torresan F, Boschin IM, et al. Early, Prophylactic Thyroidectomy in Hereditary Medullary Thyroid Carcinoma: A 26-year Monoinstitutional Experience. Am J Clin Oncol. 2015;38(5):508‒513.
- Tuggle CT, Roman SA, Wang TS, et al. Pediatric endocrine surgery: who is operating on our children? Surgery. 2008;144:869–877.
- Castagna MG, Fugazzola L, Maino F, et al. Reference range of serum calcitonin in pediatric population. J Clin Endocrinol Metab. 2015;100(5):1780‒1784.
- Siegel DA, King J, Tai E, et al. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics. 2014;134(4):945‒955.