Volume : 1 | Issue : 2
Research
Characterization of industrial effluents and groundwater of Hattar industrial estate, Haripur
Muhammad Salman Afzal, Adil Ashraf, Mohammad Nabeel
Department of Environmental Engineering, University of Engineering & Technology, Pakistan
Received: June 19, 2018 | Published: July 17, 2018
Abstract
Ground water quality of Hattar Industrial Area is deteriorating due to highly polluted industrial discharge of Hattar Industrial Estate, Haripur (Pakistan). The objective of this study was to correlate the ground water deterioration with the industries operating in Hattar Industrial Estate. It was done by evaluating various industrial effluent sand assessing the impacts of such effluents on the groundwater of the surrounding area and then, identifying the suitable method for treatment. A total of 13 samples including 6 from various industrial effluent drains (marble, textile, heavy electrical engineering, chemicals, ghee and cooking oil, food and beverages industries), 2 from main drain and 5 from tube or bore wells in the surrounding area were collected and analyzed regularly for sixmonths (Sep. 2015 to Mar. 2016). The industrial effluents and main drain samples were analyzed for temperature, color, pH, TSS, TDS, chlorides, sulfate, ammonia nitrogen, COD, BOD and heavy metals content (Ni, Cd, Pb, Cr, Cu, Zn, Fe, Mn, As and Hg).The groundwater samples were analyzed for color, taste, odor, pH, EC, turbidity, alkalinity, hardness, DO, TSS, TDS, chloride, sulfate, phosphate, nitrate, ammonia nitrogen, sodium, calciumand heavy metals content (Ni, Cd, Pb, Cr, Cu, Zn, Fe, Mn, As and Hg). The analysis of industrial effluents and groundwater in the vicinity of industrial estate concluded that the effluents were highly polluted due to the presence of heavy metals and hence, contemplated as ill-suited for irrigation and drinking purposes. Coagulation flocculation was therefore recommended for the treatment of industrial effluents.
Keywords: characterization, groundwater contamination, coagulation, flocculation
Introduction
Water is very important for life to exist. The one of the most important environmental issue of the 21st century is the availability of clean and quality freshwater.1 In Pakistan per capita water availability was 5,300m3 per person per year in 1951, which decreased to less than 1,100m3 per person per year in 2007 due to population growth and is expected to be not more than 700m3 per person per year by 2025. Therefore, Pakistan has reached the withdrawal limits of its surface and groundwater sources.2 Pakistan is heading certainly into the category of water scarce countries, defined as having water availability less than 1,000m3 per person per year.3
Nature provides water which is the most important component for the life of plants and animals. Also, water plays an important role in every mode of human life.4 Despite of all benefits, it is the most poorly managed resource.5 Thoughtless disposal of industrial effluents and other harmful wastes contribute to the poor quality of surface as well as groundwater. This is the most important and critical environmental issue that is posing damage to the survival of human life.5,6 The presence of organic constituents, pathogens, heavy metals and other trace elements in water bodies results in the pollution of water resources. Presence of heavy metals in effluent discharges has an adverse impact on the environment of water. Discharge of heavy metals deteriorates the water quality by changing the properties of water such as pH, EC, TDS etc and thus, has adverse negative impact on human life.5
Groundwater is an important source of drinking water throughout the world. Urbanization, industrialization and unregulated population growth affects the groundwater resource, both qualitatively and quantitatively.7 Pure water is a limited, precious and renewable natural resource which cannot be produced synthetically. Rapid increase in population has exerted a continuous thrust on this limited natural resource.8 Simultaneously, the constantly increasing industrialization and ever expanding urbanization have considerably increased the rate of groundwater pollution. The pollution of groundwater resource due to devastating industrial activities has threatened the existence of human beings including other organisms that relies on freshwater resources for their survival. The crises are more severe in developing countries like Pakistan.9 The major source of groundwater pollution is the discharge of untreated industrial effluents.10,11
Groundwater is often considered as a reliable source of fresh water which is easily accessible. Generally, groundwater is pure than surface water.12 At the same time, the economic up gradation has resulted in severe impacts on groundwater characteristics. The indiscriminate disposal of industrial effluents and leachates from landfills had caused considerable decline in the groundwater quality.13
Surface and groundwater sources are very much influenced by natural and manmade processes, resulting in the raised levels of different impurities in drinking water.14,15 Numerous pathogens along with organic and inorganic components occurs in drinking water source sand all these contaminants can have acute and chronic effects on users’ health.16 For instance, one-third of annual deaths in Pakistan has been credited to drinking water contamination by microbial or chemical components.17 Trace metals are recognized as an important group of contaminants of drinking water sources that may have significant consequences on human health, e.g., skeletal and cardiovascular diseases, neurotoxicity and infertility.18 During the recent few decades, industrialization and excessive use of pesticide sand chemical fertilizers in the under developed countries resulted in contamination of drinking water sources.16,19,20 Due to insufficient mitigation measures, water contaminated with trace metals is frequently being consumed in the developing countries.21,22
The purpose of this study was to associate the groundwater deterioration with the industries established in Hattar Industrial Estate. Various industrial effluents were evaluated and their impacts on groundwater of the surrounding area were assessed. Suitable treatment method was then identified based on the estimated results.
Materials and methods
Study area
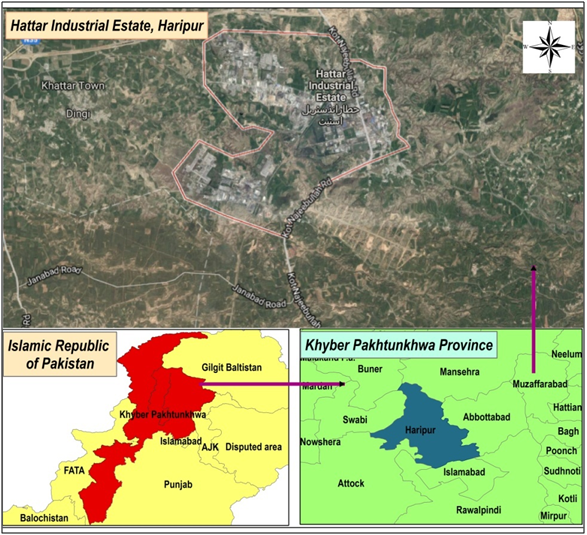
The study was carried out in Hattar Industrial Estate located in Haripur Basin. Hattar Industrial Estate is one of the largest industrial estates of the Province Khyber Pakhtunkhwa (KP). The total area of the estate is 1,063acres. There are more than 360 industries of various kinds which includes textile, chemicals, marble, heavy electrical engineering, ghee & cooking oil, food & beverages industries.23,24 The population present in the vicinity of Hattar Industrial Estate is dependent on groundwater resources. Therefore, monitoring of water resources and pollution control is a mandatory requirement.25,26 Most of the industries present in the industrial estate discharge their effluents directly or indirectly into the drain without any treatment, which sinks into Chahari Kas stream and then to Jabbi Kas and finally to Haro River. The key location map of study area of Hattar Industrial Estate is shown in Figure 1.
Field investigations
Sample collection: Field survey was conducted for identification of sampling points. To collect a representative sample, five composite samples were collected at one hour interval. The effluents drain was kept under observation for sixmonths (Sep. 2015 to Mar. 2016) and was sampled with 2days interval for 2months, then with 4days interval for next 2months and in the end, 7days interval for last 2months.
Surface water sampling: Eight surface water samples namely F1, F2, F3, F4, F5, F6, F7, F8 were collected from the selected points of Hattar Industrial Area including 6 samples from various industrial effluent drains (marble, textile, heavy electrical engineering, chemicals, ghee & cooking oil, food & beverages industries) and 2 from main drain regularly for sixmonths.
Groundwater sampling: Five groundwater samples namely G1, G2, G3, G4, G5 were collected from Hattar Industrial Area regularly for sixmonths. Groundwater samples G1, G2, G3, G4 and G5 were collected approximately 300m away from Jabbi Kas Stream, 100m from Chahri Kas Stream, 150m from Dhotal Kas Stream, 50m from Chahri Kas Stream and from 400m away from Dhotal Kas Stream respectively. Also, samples G1, G2, G3, G4 and G5 were collected from bore holes, tube wells with depths approximately 60 ft, 70 ft, 80 ft, 50 ft and 65 ft respectively.
Analytical procedures
The industrial effluents and main drain samples were characterized for 10 wastewater quality parameters which included temperature, color, pH, TSS, TDS, chloride, sulfate, ammonia nitrogen, COD, BOD and heavy metals content (Ni, Cd, Pb, Cr, Cu, Zn, Fe, Mn, As and Hg). The groundwater samples were characterized for 17 water quality parameters, incorporating color, taste, odor, pH, EC, turbidity, alkalinity, hardness, DO, TSS, TDS, chloride, sulfate, phosphate, nitrate, ammonia nitrogen, sodiumand heavy metals content (Ni, Cd, Pb, Cr, Cu, Zn, Fe, Mn, As and Hg).
All the tests were carried out in the Laboratories of Environmental Engineering Department, UET, Taxila. All the samples were analyzed according to the procedures laid down in “Standard Methods for the Examination of Water and Wastewater”.27The heavy metals present in samples were determined by using Atomic Absorption Spectro-photometric (AAS) method and Spectrophotometer. The spectrophotometer was used for the determination of Zn and Fe, while, Ni, Cd, Pb, Cr, Cu, Mn, As and Hg were measured through AAS. The objective was to evaluate the pollution degree by the analysis of various parameters which characterized industrial effluents and groundwater samples.28
Coagulation and flocculation
Coagulation-flocculation tests were performed on jar test apparatus as per standard procedure. There were six jars, each had a capacity of one liter and were equipped with a sampling port for sampling of the treated effluent. Standardized mixing speeds and durations were used. For each jar test, 1-L of wastewater sample was taken in the jars and rapidly mixed at 300rpm for 3min. The required doses of the coagulant were added to the jars during the rapid mixing.29 The rapid mix was followed by slow flocculation at 30rpm for 15min. A settling time of 30min was also given before drawing the sample. Jar number 1 in all the jar tests was used as a control jar or “zero coagulant jar”. The coagulant was not added to this jar to simulate plain sedimentation. By using above methodology of jar test apparatus, optimum dosage of alum, ferric chloride and natural coagulant named fruit of Opuntia Microdasys commonly called bunny ears was determined.30
Results and discussion
The main source of groundwater pollution is the polluted industrial effluents. To evaluate the pollution level, eight samples from different industries and main drains were analysed for various wastewater quality parameters. Heavy metals content was also analysed in industrial effluents and main drains samples. The results were compared with National Environmental Quality Standards (NEQS) for industrial effluents. In addition to this groundwater samples were evaluated and the values were compared with standards of United States Environmental Protection Agency (USEPA), World Health Organization (WHO) and also with National Environmental Quality Standards (NEQS) for drinking water.
Industrial effluents analysis
Comparison of average values of industrial effluents and main drain samples with National Environmental Quality Standards (NEQS) for Industrial Effluents is shown in the Table 1.
Parameters |
Sampling points |
NEQS |
Analytical methods |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
|||
Wastewater quality parameters |
||||||||||
Temperature(◦C) |
19.9 |
28.5 |
33.9 |
41 |
21.4 |
27.4 |
23.5 |
35.5 |
40 |
Thermometer |
Colour |
White |
Light Blue |
Light Brown |
Brown |
Light Brown |
Yellow |
Black |
Light Brown |
- |
Visual |
PH |
8.07 |
7.5 |
6.9 |
6.5 |
7.8 |
8.95 |
7.66 |
7.26 |
6.0 - 10.0 |
Digital PH Meter |
TSS(mg/L) |
1,980 |
249 |
922 |
521 |
436 |
798 |
1,412 |
1,540 |
150 |
Gravimetric |
TDS(mg/L) |
288 |
287 |
640 |
4,900 |
290 |
1,588 |
1,740 |
2,788 |
3,500 |
TDS Meter |
Chloride(mg/L) |
188 |
154 |
158 |
212 |
235 |
179 |
451 |
431 |
1,000 |
Mercuric Nitrate Method |
Sulphate(mg/L) |
147 |
254 |
423 |
254 |
346 |
212 |
945 |
934 |
600 |
Turbidity Method |
Ammonia Nitrogen(mg/L) |
125 |
136 |
173 |
87 |
65 |
76 |
122 |
101 |
40 |
Kjeldahl Apparatus |
COD(mg/L) |
412 |
177 |
195 |
212 |
321 |
379 |
890 |
940 |
150 |
Closed Reflux Method |
BOD(mg/L) |
180 |
115 |
212 |
189 |
120 |
77 |
450 |
415 |
80 |
Dilution Media Method |
Heavy metals content |
||||||||||
Ni(mg/L) |
0.29 |
0.45 |
0.88 |
1.62 |
0.62 |
0.59 |
1.25 |
1.52 |
1 |
AAS |
Cd(mg/L) |
0.13 |
0.22 |
0.11 |
0.32 |
0.12 |
0.05 |
0.35 |
0.15 |
0.1 |
AAS |
Pb(mg/L) |
1.38 |
0.88 |
0.43 |
3.71 |
0.65 |
0.27 |
0.91 |
0.88 |
0.5 |
AAS |
Cr(mg/L) |
0.45 |
0.78 |
0.32 |
0.15 |
0.99 |
0.89 |
0.88 |
0.91 |
1 |
AAS |
Cu(mg/L) |
0.89 |
0.35 |
0.49 |
0.12 |
0.99 |
0.21 |
1.12 |
1.34 |
1 |
AAS |
Zn(mg/L) |
0.05 |
0.22 |
0.33 |
3.01 |
0.11 |
0.06 |
3.2 |
3.99 |
5 |
Spectrophotometer |
Fe(mg/L) |
0.78 |
0.55 |
0.89 |
0.99 |
1.5 |
0.04 |
1.95 |
1.6 |
2 |
Spectrophotometer |
Mn(mg/L) |
0.24 |
0.35 |
0.56 |
0.99 |
0.56 |
0.11 |
1.1 |
0.98 |
1.5 |
AAS |
As(mg/L) |
2.2 |
0.87 |
2.2 |
1.56 |
1.12 |
2.2 |
5.5 |
4.2 |
1 |
AAS |
Hg(mg/L) |
0.18 |
0.32 |
0.22 |
0.35 |
0.17 |
0.45 |
0.54 |
0.65 |
0.01 |
AAS |
Table 1 Comparison of average values of industrial effluents and main drain samples with National Environmental Quality Standards(NEQS) for industrial effluents
F1=Marble Industry, F2=Textile, F3=Heavy Electrical Engineering, F4=Chemicals, F5=Ghee & Cooking Oil, F6=Food & Beverages, F7 & F8=Main DrainTemperature: Temperature values for various samples of wastewater are given in Table 1. The values ranged from 19.9 ◦C to 41 ◦C. The highest temperature was found in the chemical industry effluent and the lowest temperature was found in marble industry effluent drain. All the values were found to be in compliance with NEQS limit except for the chemical industry effluent.
Colour: The samples’ colour varied a lot from white for marble industry to brown for chemical industry as shown in the Table 1.
PH: The PH of the wastewater samples ranged from 6.5 to 8.95 and is presented in Table 1. The highest PH was found in the sample of food & beverage industry and the lowest PH was found in chemical industry sample. All the values of PH were found to be in compliance with the NEQS limit for industrial effluents.
Total Suspended Solids (TSS): The values of TSS (Table 1) ranged from 249 to 1,980mg/L. All the values of TSS exceeded the NEQS limit for TSS which is given as 150mg/L. The marble industry had the highest concentration of suspended solids while the lowest concentration was found in the sample of textile industry. Effluents with such high concentration of TSS will cause water pollution if it is directly discharged into the water body.
Total Dissolved Solids (TDS): The TDS concentration in the samples ranged from 288 to 4,900mg/L (Table 1). The highest value of TDS was found in the chemical industry effluent and the lowest concentration was found in the effluent of marble industry. The TDS concentration in the effluent of chemical industry exceeded the NEQS limit for TDS i.e., 3,500mg/L. All the other values for TDS were found to be in line with the limit set by NEQS for industrial effluents.
Chloride: The chloride concentration in all the wastewater samples was found in compliance with the NEQS limit (i.e., 1,000mg/L) for industrial effluents (Table 1). The textile industry effluent had the lowest concentration of chloride and the main drain sample had the highest concentration.
Sulphate: The sulphate values of samples ranged from 147 to 945mg/L (Table 1). The highest concentration of sulphate was found in the main drain and the lowest was found in the effluent of marble industry. All the values of industrial effluents were found to be in compliance with the NEQS limit except the main drains samples.
Ammonia Nitrogen: The values of NH3-N ranged from 65 to 173mg/L and are presented in Table 1. All the values were found to be above the permissible limit of NEQS for industrial effluents.
Chemical Oxygen Demand (COD): The COD values ranged from 177-940mg/L. The COD values for different wastewater samples are given in Table 1. The highest value of COD was found in the main drain sample while the lowest was found in the effluent of textile industry. All the values exceeded the NEQS limit i.e., 150mg/L.
Biological Oxygen Demand (BOD): The BOD values of samples ranged from 77 to 450mg/L (Table 1). The highest value of BOD was recorded in the main drain sample and the lowest was recorded in the effluent of food and beverage industry. All the values exceeded the NEQS limit i.e., 80mg/L except the value of food and beverage industry.
Heavy metals: The heavy metals content of various samples is presented in Table 1. The nickel (Ni) concentration ranged from 0.29 to 1.62mg/L. The NEQS limit for Ni (i.e., 1.0mg/L) was violated by chemical industry and main drain samples. The concentration of cadmium (Cd) ranged from 0.05 to 0.35mg/L. All the values of Cd exceeded the NEQS limit (i.e., 0.1mg/L) except that for food & beverage industry. The value of lead (Pb) ranged from 0.27 to 3.71mg/L for the wastewater samples. The highest value of Pb was found in the effluent of chemical industry. The Pb concentration of all the samples exceeded the NEQS limit (i.e., 0.5mg/L) except for food & beverage industry. The concentration of chromium (Cr) of all the samples was found to be in compliance with the NEQS limit (i.e., 1.0mg/L). The copper (Cu) concentration ranged from 0.12-1.34mg/L. The Cu concentration of all the samples was found to be in compliance with the NEQS limit (i.e., 1.0mg/L) except the main drain samples. The zinc (Zn) concentration of all the samples was found to be within the permissible limit of NEQS (i.e., 5.0mg/L). Similarly, the concentration of iron (Fe) for all the samples was found to be within the permissible limit of NEQS (i.e., 2.0mg/L). The manganese (Mn) concentration ranged from 0.24 to 1.1mg/L. All the values of Mn were found to in compliance with the limit of NEQS (i.e., 1.5mg/L). The values of arsenic (As) ranged from 0.87 to 5.5mg/L. The concentration of As for all the samples exceeded the NEQS limit for As (i.e., 1.0mg/L) except that for the effluent of textile industry. The concentration of mercury (Hg) in all the wastewater samples was found to be high above the limit set by NEQS for industrial effluents (i.e., 0.01mg/L).
Groundwater analysis
Comparison of average values of groundwater samples with USEPA, WHO and NEQS standards for drinking water is shown in the Table 2.
Parameters |
Sampling points |
Mean |
SD |
USEPA Standards |
WHO Standards |
NEQS |
Analytical methods |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
G1 |
G2 |
G3 |
G4 |
G5 |
|||||||
Water quality parameters |
|||||||||||
Colour |
Light Brown |
Light Brown |
Brown |
Light Brown |
Light Brown |
- |
- |
Colourless |
Colourless |
Colourless |
Visual |
Odour(TON) |
1 |
2 |
2 |
3 |
3 |
2.2 |
0.8 |
Odourless |
Odourless |
Odourless |
Quantitative |
PH |
7.65 |
7.54 |
7.88 |
7.81 |
7.71 |
7.7 |
0.1 |
6.0 - 8.5 |
6.5 - 8.5 |
6.5 - 8.5 |
PH Meter |
EC(µS/cm) |
353 |
390 |
295 |
312 |
240 |
318 |
57.1 |
- |
- |
- |
Turbidity Meter |
Turbidity(NTU) |
23 |
25 |
37 |
32 |
27 |
28.8 |
5.7 |
300 |
400 |
- |
Titration |
Alkalinity(mg/L) |
124 |
96 |
112 |
43 |
64 |
87.8 |
33.7 |
- |
- |
- |
Titration |
Hardness(mg/L) |
345 |
564 |
576 |
458 |
347 |
458 |
112.1 |
- |
- |
- |
EDTA Titrimetric Method |
DO(mg/L) |
4 |
5 |
4.5 |
6 |
6 |
5.1 |
0.9 |
4 |
3 |
- |
DO Meter |
TSS(mg/L) |
2.03 |
2.19 |
1.85 |
2.11 |
1.97 |
2 |
0.1 |
0 – 5 |
5 |
- |
Gravimetric |
TDS(mg/L) |
353 |
390 |
294 |
314 |
335 |
337.2 |
36.9 |
500 |
500 |
- |
TDS Meter |
Chloride(mg/L) |
121 |
102 |
78 |
76 |
86 |
92.6 |
18.9 |
250 |
250 |
< 250 |
Titration |
Sulphate(mg/L) |
159 |
254 |
468 |
357 |
346 |
316.8 |
116.4 |
250 |
200 – 400 |
- |
Turbidity Method |
Phosphate(mg/L) |
1.2 |
0.6 |
0.2 |
0.64 |
1.4 |
0.8 |
0.5 |
- |
- |
- |
Spectrophotometer |
Nitrate(mg/L) |
1.53 |
2.54 |
3.5 |
2.4 |
2.34 |
2.5 |
0.7 |
100 |
50 |
< 50 |
Spectrophotometer |
Ammonia Nitrogen(mg/L) |
98 |
35 |
67 |
76 |
86 |
72.4 |
23.9 |
- |
- |
- |
Kjeldahl Apparatus |
Sodium(mg/L) |
45 |
23 |
34 |
17 |
32 |
30.2 |
10.8 |
20 |
200 |
- |
Flame Photometer |
Calcium(mg/L) |
46 |
35 |
75 |
48 |
42 |
49.2 |
15.3 |
100 |
100 |
- |
Flame Photometer |
Heavy metals content |
|||||||||||
Ni(mg/L) |
0.48 |
0.67 |
0.45 |
0.73 |
0.88 |
0.7 |
0.2 |
0.01 |
0.02 |
< 0.02 |
AAS |
Cd(mg/L) |
0.06 |
0.04 |
0.02 |
0.04 |
0.01 |
0.04 |
0.02 |
0.01 |
0.003 |
0.01 |
AAS |
Pb(mg/L) |
0.07 |
0.43 |
0.88 |
0.2 |
0.41 |
0.6 |
0.4 |
0.05 |
0.01 |
< 0.05 |
AAS |
Cr(mg/L) |
0.04 |
0.17 |
0.06 |
0.15 |
0.05 |
0.1 |
0.1 |
0.1 |
0.05 |
< 0.05 |
AAS |
Cu(mg/L) |
0.45 |
0.41 |
0.32 |
0.34 |
0.35 |
0.4 |
0.1 |
0.05 |
2 |
2 |
AAS |
Zn(mg/L) |
0.08 |
0.24 |
0.99 |
1.02 |
1.54 |
1 |
0.6 |
5 |
5 |
3 |
Spectrophotometer |
Fe(mg/L) |
0.25 |
0.02 |
0.07 |
0.06 |
0.04 |
0.1 |
0.1 |
0.3 |
0.3 |
- |
Spectrophotometer |
Mn(mg/L) |
0.23 |
0.09 |
0.12 |
0.07 |
0.06 |
0.1 |
0.1 |
0.05 |
0.5 |
< 0.5 |
AAS |
As(mg/L) |
0.32 |
2.1 |
0.76 |
2.5 |
1.56 |
1.6 |
0.9 |
0.05 |
0.01 |
< 0.05 |
AAS |
Hg(mg/L) |
0.19 |
0.22 |
0.21 |
0.19 |
0.32 |
0.3 |
0.1 |
0.002 |
0.001 |
< 0.001 |
AAS |
Table 2 Comparison of average values of groundwater samples with USEPA, WHO and NEQS standards for drinking water
Colour: All the groundwater samples were found to be light brown and brown in colour as presented in Table 2.
Odour: The values of odour for groundwater samples ranged from 1.0 TON to 3.0 TON. The mean value of odour was worked out to be 2.2 TON with the standard deviation of 0.8 TON (Table 2).
PH: The average PH value of five different groundwater samples was 7.7. PH values of samples are shown in Table 2. The mean value was found to be within the permissible limits of USEPA, WHO and NEQS standards.
Electrical Conductivity (EC): EC values of groundwater samples shown in Table 2. The mean value of EC for all five groundwater samples was 318µS/cm. The mean value was found to be within the limits of USEPA and WHO standards for drinking water.
Turbidity: The mean value of turbidity was found to be 28.8 NTU with the deviation of 5.7 NTU as shown in Table 2.
Alkalinity: Alkalinity values for groundwater samples ranged from 43 to 124mg/L, as presented in Table 2. The average value of five samples is 87.8mg/L.
Hardness: The hardness of samples ranged from 345 to 576mg/L (Table 2). The mean value of five groundwater samples was 458mg/L.
Dissolved Oxygen (DO): DO of samples ranged from lowest value of 4.0mg/L to highest value of 6.0mg/L with the mean value of 5.1mg/L, as presented in Table 2. The values were found to be in compliance with theminimum level of DO for drinking water according to USEPA and WHO standards.
Total Suspended Solids (TSS): The concentration of TSS in the samples ranged from 1.85 to 2.19mg/L (Table 2). The mean value of TSS of five groundwater samples was found out 2.0mg/L. All the values were found to be well below the standards of USEPA and WHO for drinking water.
Total Dissolved Solids (TDS): The TDS values were found to be well below the USEPA and WHO standards i.e., 500mg/L. The mean value of TDS was evaluated as 337.2mg/L with the standard deviation of 36.9mg/L (Table 2).
Chloride: The chloride concentration in the samples ranged from 76 to 121mg/L (Table 2). All the values were found to be well below the limits set for drinking water by USEPA, WHO and NEQS.
Sulphate: The concentration of sulphate ranged from 159 to 357mg/L and is presented in Table 2. The sulphate value of four samples was found to be above the permissible limit set by USEPA i.e., 250mg/L. The three samples of groundwater had sulphate values that were found to be above the WHO standards of sulphate i.e., 200-400mg/L for drinking water.
Phosphate:The values of phosphate ranged from 0.2 to 1.2mg/L (Table 2). The mean value is 0.8mg/L with the standard deviation of 0.5mg/L.
Nitrate: The nitrate values ranged from 1.53 to 3.5mg/L as presented in Table 2. The mean value of nitrate in five groundwater samples was worked out to be 2.5mg/L. All the values were found within the permissible limits of USEPA, WHO and NEQS.
Ammonia Nitrogen: The NH3-N concentration in the groundwater samples lies between 35 and 98mg/L. The mean value is 72.4mg/L with the standard deviation of 23.9mg/L (Table 2).
Sodium: The values of sodium in the samples ranged from 17 to 45mg/L and are presented in Table 1. The sodium concentration in three out of five samples was found to be well below the USEPA standards. All the values were found to be in compliance with the WHO standards for drinking water.
Calcium: All the values of calcium were found to be within the permissible limits of USEPA and WHO (Table 2).
Heavy metals: The heavy metals content of five groundwater samples is given in Table 2.
- The values of Ni, Cd, Pb, Cu, Mn, As and Hg were exceeding the USEPA standards while the concentration of Cr, Zn and Fe were found to be well below the USEPA standards.
- The values of Cu, Zn, Fe and Mn were found to be below the WHO standards. All other values were above the standards set by WHO for drinking water.
- The NEQS standards for drinking water were violated in case of Ni, Cd, Pb, As and Hg. The values of Cu, Zn and Mn were found to be within the limits of NEQS for drinking water.
Treatment
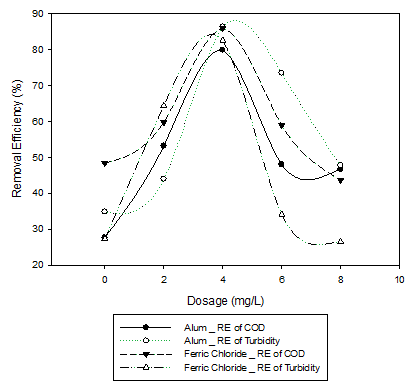
The results of jar test have been graphically presented here. Alum, ferric chloride and natural coagulant named fruit of Opuntia Microdasys commonly called bunny ears used as coagulant. Standard method of Jar test apparatus was used for this purpose. The optimum dosage of alum was 4mg/L with removal efficiency of COD and turbidity of about 80% and 86% respectively (Graph 1). The optimum dosage for the removal efficiency of COD and turbidity of 86% and 83% respectively was 2mg/L of ferric chloride (Graph 1).
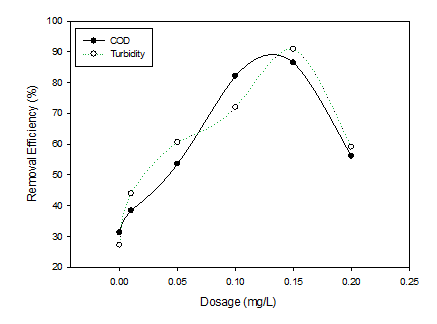
The optimum dosage of natural coagulant was found out to be 0.15mg/L for the removal of 86% of COD and 90% of turbidity from the wastewater sample (Graph 2). The optimum dosage of alum, ferric chloride and natural coagulant and Influent/Effluent values of various parameters are shown in Table 3.
Sr. No. |
Coagulants |
Optimum Dosage |
Influent COD(mg/L) |
Effluent COD(mg/L) |
Influent Turbidity(NTU) |
Effluent Turbidity(NTU) |
Influent Nickel(mg/L) |
Effluent Nickel(mg/L) |
Influent Cadmium(mg/L) |
Effluent Cadmium(mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
1 |
Alum |
4 mg/L |
940 |
189 |
132 |
18 |
1.25 |
0.65 |
0.35 |
0.01 |
2 |
Ferric Chloride |
3 mg/L |
940 |
132 |
132 |
23 |
1.25 |
0.6 |
0.35 |
0.005 |
3 |
Natural |
0.15 mg/L |
940 |
127 |
132 |
12 |
1.25 |
0.5 |
0.35 |
0.001 |
Sr. No |
Coagulants |
Optimum Dosage |
Influent Lead(mg/L) |
Effluent Lead(mg/L) |
Influent Chromium(mg/L) |
Effluent Chromium(mg/L) |
Influent Copper(mg/L) |
Effluent Copper(mg/L) |
Influent Zinc(mg/L) |
Effluent Zinc(mg/L) |
1 |
Alum |
4 mg/L |
0.91 |
0.5 |
1.12 |
0.9 |
1.12 |
0.98 |
3.2 |
2.9 |
2 |
Ferric Chloride |
3 mg/L |
0.91 |
0.3 |
1.12 |
0.76 |
1.12 |
0.65 |
3.2 |
2.4 |
3 |
Natural |
0.15 mg/L |
0.91 |
0.1 |
1.12 |
0.54 |
1.12 |
0.43 |
3.2 |
1.9 |
Sr. No. |
Coagulants |
Optimum Dosage |
Influent Iron(mg/L) |
Effluent Iron(mg/L) |
Influent Manganese(mg/L) |
Effluent Manganese(mg/L) |
Influent Arsenic(mg/L) |
Effluent Arsenic(mg/L) |
Influent Mercury(mg/L) |
Effluent Mercury(mg/L) |
1 |
Alum |
4 mg/L |
1.95 |
1.65 |
1.1 |
0.989 |
5.5 |
1.5 |
0.54 |
0.2 |
2 |
Ferric Chloride |
3 mg/L |
1.95 |
1.23 |
1.1 |
0.767 |
5.5 |
1.1 |
0.54 |
0.11 |
3 |
Natural |
0.15 mg/L |
1.95 |
0.98 |
1.1 |
0.521 |
5.5 |
0.89 |
0.54 |
0.05 |
Table 3 Optimum doses of different coagulants and Influent/Effluent values of various parameter
Conclusion
The discharge of untreated industrial effluents into the surface water bodies is a major source of surface as well as groundwater pollution. The analysis of industrial effluents and groundwater of the surrounding area of Hattar Industrial State concluded that the effluents were highly polluted with heavy metals, degrading the groundwater quality and making it unfitted for irrigation purpose. Furthermore, the groundwater in the vicinity of industrial estate was immensely polluted by industrial effluents and considered as unsuitable for drinking. Coagulation flocculation was thus recommended for the treatment of industrial effluents.
Recommendations
- Currently, industries in Hattar Industrial Estate are discharging untreated effluents in the main drains which eventually fall into the Haro River.
- Identify the industries in Hattar Industrial Estate that are the biggest contributor to surface and groundwater pollution.
- The National Environmental Quality Standards (NEQS) for industrial effluents should be strictly enforced.
- The industries that are the biggest polluters of water bodies should be directed to install proper wastewater treatment plant.
- There should be common effluents treatment plant for the industrial estate to treat the industrial effluents.
- Khyber Pakhtunkhwa Environmental Protection Agency (KP-EPA) should ensure the regular monitoring of the estate to improve the environmental condition of the area.
- Awareness should be created among the industries for environmental friendly measures.
Acknowledgement
None.
References
- Mohamed Hanipha M, Zahir Hussain A. Study of groundwater quality at Dindigul town, Tamilnadu, India. International Research Journal of Environment Sciences. 2013;2(1):68‒73.
- Haroon H, Waseem A, Mahmood Q. Treatment and reuse of wastewater from beverage industry. J Chem Soc Pak. 2013;35(1):5‒10.
- Zeb S, Waseem A, Mahmood Q. International Journal of Physical Sciences. 2011:6(7789).
- Nwankwoala H, Nwagbogwu C. Characteristics and quality assessment of groundwater in parts of Akure, South-Western Nigeria. Journal of Environmental Science and Water Resources. 2012;1(4):67‒73.
- Islam M. Effects of solid waste and industrial effluents on water quality of Turag River at Konabari industrial area, Gazipur, Bangladesh. Journal of Environmental Science and Natural Resources. 2012;5(2):213‒218.
- Rahman MM, Sultana KR, Hoque MA. Suitable sites for urban solid waste disposal using GIS approach in Khulna city, Bangladesh. Proceedings of the Pakistan Academy of Sciences (Pakistan). 2008.
- Vasanthavigar M. Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environmental monitoring and assessment. 2010;171(1‒4):595‒609.
- Watson PS, Davies S. Modeling the effects of population growth on water resources: a CGE analysis of the South Platte River Basin in Colorado. The Annals of Regional Science. 2011;46(2):331‒348.
- Arora T. Human health risk assessment of temporal and spatial variations of ground water quality at a densely industrialized commercial complex at Haridwar, India. Journal of Applied and Natural Science. 2014;6(2):825‒843.
- Singh R. Effect of wastewater disposal and extent of industrial pollution in and around Kanpur, Uttar Pradesh, India. Bulletin of Engineering Geology and the Environment. 2001;60(1):31‒35.
- Xu B. Occurrence and health risk assessment of trace heavy metals via groundwater in Shizhuyuan Polymetallic Mine in Chenzhou City, China. Frontiers of Environmental Science & Engineering. 2015;9(3):482‒493.
- Saravi M, Keshavarzi A, Azareh A. Modeling of SAR and sulfate concentration using artificial neural network approach. International Journal of Agronomy and Plant Production. 2013;4(3):499‒506.
- Nagarajan R, Thirumalaisamy S, Lakshumanan E. Impact of leachate on groundwater pollution due to non-engineered municipal solid waste landfill sites of erode city, Tamil Nadu, India. Iranian journal of environmental health science & engineering. 2012;9(1):35.
- Winkel L. Predicting groundwater arsenic contamination in Southeast Asia from surface parameters. Nature Geoscience. 2008;1(8):536‒542.
- Shah M. Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohmand agency, northern Pakistan. Journal of Geochemical Exploration. 2012;118:60‒67.
- Gowd SS, Govil PK. Distribution of heavy metals in surface water of Ranipet industrial area in Tamil Nadu, India. Environmental monitoring and assessment. 2008;136(1‒3):197‒207.
- Azizullah A. Water pollution in Pakistan and its impact on public health a review. Environment International. 2011;37(2):479‒497.
- Organization WH. Guidelines for drinking-water quality: recommendations. Vol 1. World Health Organization. 2004.
- Eqani SAMAS. Status of organochlorine contaminants in the different environmental compartments of Pakistan: a review on occurrence and levels. Bulletin of environmental contamination and toxicology. 2012;88(3):303‒310.
- Farooqi A. Sources of arsenic and fluoride in highly contaminated soils causing groundwater contamination in Punjab, Pakistan. Archives of environmental contamination and toxicology. 2009;56(4):693‒706.
- Ullah R, Malik RN, Qadir A. Assessment of groundwater contamination in an industrial city, Sialkot, Pakistan. African Journal of Environmental Science and Technology. 2009;3(12).
- Khan S. Drinking water quality and human health risk in Charsadda district, Pakistan. Journal of cleaner production. 2013;60:93‒101.
- Pakistan Go. Department of planning and development, Sarhad development authority (SDA), Pakistan; 2009.
- Hussain, R. Optimization of wastewater treatment process in industry a case study of Hattar Industrial Estate Haripur. Pak J Anal Environ Chem. 2014;15(1):28‒34.
- Manzoor S. Studies on Chemical Profiling of Wastewater from Hattar Industrial Estate (NWFP), Pakistan. Islamabad: Quaid-i-Azam University; 2008.
- Hussain K, Shahazad A, Zia-ul-Hussnain S. An ethnobotanical survey of important wild medicinal plants of Hattar districtHaripur, Pakistan. Ethnobotanical Leaflets. 2008;12:29‒35
- Rice EW, Bridgewater L, Association APH. Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association; 2012.
- Hazourli S. Pollution characterization of waste water of an industrial zone example of a dairy water clarification. Lebanese Science Journal. 2009;10(2):17‒31.
- Haydar S, Aziz JA. Coagulation–flocculation studies of tannery wastewater using combination of alum with cationic and anionic polymers. Journal of Hazardous Materials. 2009;168(2‒3):1035‒1040.
- Zeng Y. Feasibility investigation of oily wastewater treatment by combination of zinc and PAM in coagulation/flocculation. Journal of hazardous materials. 2007;147(3):991‒996.