Volume : 1
Case Report
Systemic treatment of tumors of the central nervous system: case report and extensive literature review
Rossio Medina Barrionuevo,1 Henry Luis Jorge Barroso,2 Alejandro Ibañez,3 Luis Medina Pérez4
1Medical oncologist of Private Banking Hospital and Social Security Hospital of La Paz, Bolivia
2Neurosurgeon specialist in Endo-vascular therapy of Social Security Hospital of La Paz, Bolivia
3Neurosurgeon, Hospital director of Private Banking Hospital of La Paz, Bolivia
4Surgeon Oncologist, General Hospital La Paz, Bolivia
Received: July 23, 2018 | Published: August 02, 2018
Summary
Neoplasms of the central nervous system (CNS) make up less than 2% of all tumors in humans. However, they represent a real public health problem because it affects mainly people of productive age, with a mostly reserved forecast. Because they constitute a heterogeneous group of neoplasms with a variety of evolution and treatment strategies, the prognostic factors and treatment options must be carefully reviewed by a multidisciplinary group of management experts. In the present article, 4 cases of gliomas with different evolution and prognosis are described, in addition to the personalized treatment options.
Keywords: Low and high grade astrocytoma, point mutations, chemotherapy
Introduction
Neoplasms of the central nervous system (CNS) make up less than 2% of all tumors in humans. However, they represent a real public health problem because it affects mainly people of productive age. For 2016, an estimated 23770 people in the US have been diagnosed with primary tumors of the CNS, of which these tumors are responsible for approximately 16050 deaths per year. That is, practically more than 50% of patients diagnosed with tumors of the central nervous system will end up with a fatal outcome.1
There is a variation of survival according to the histological type, with pilocytic astrocytoma having a 5-year overall survival of around 92%, while Glioblastoma multiforme is so lethal that less than 5% survive to 5 years, grade astrocytomas II around 50 to 70% and anpalsic astrocytoma only 37%.1,2
Presentation of cases
Case 1
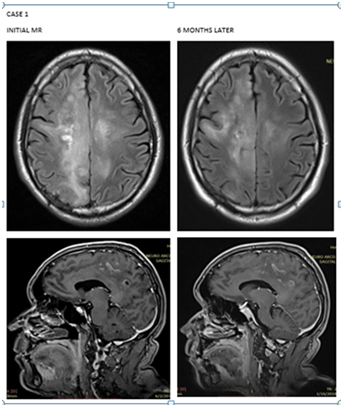
A 52-year-old male patient with a 1-month history of evolution with paresthesias in the left pelvic limb that subsequently evolved to left hemiparesis with brachial predominance 2/5, crural 3/5 and seizures. Brain MRI shows Heterogenicity of the brain parenchyma, evidencing extensive neoformative process mainly at the medial level, compromising both sides of the midline. Showing areas of linear hyperintensity with predominance of right parietal convexity and predominance of hypointensity. In sequences weighted in T2, it shows diffuse hyperintensity. It presents pseudonodular areas that are located towards the right occipito-parietal region. It is associated with the presence of discrete thickening of the corpus callosum mainly at the level of splenium and body, showing slight heterogeneity. It presents moderate perilesional edema with a cytotoxic appearance, associated with mild effacement of fissures at the level of the right convexity. Stereotactic biopsy was performed with oligodendroglioma report with positive IDH1 mutation and co-deleccion 1p19q. By age, extension of the disease and neurological deterioration at presentation, it is considered high risk so 60 Gy radiotherapy is started in 30 fractions plus chemotherapy concomitant with temozolamide and then adjuvant temozolamide every 4 weeks for 6 cycles. MRI control of brain with stable disease. It is currently 19 months after diagnosis with total recovery of crural and brachial muscle strength. Due to the presentation of the disease, it is decided to maintain temozolamide every 4 weeks indefinitely (Figure 1).
Case 2
A 28-year-old female patient with cervical intramedullary tumor and quadriparesis with predominance of brachial left 1/5, crural 2/5, right brachial and crural 3/5 by MRI with intradural lesion extending from C4 to C5 of heterogeneous characteristic. Initially, a partial tumor resection of 60% was performed with a report of pathology indicating astrocytoma grade II of the WHO, it could not perform IDH mutations and co-deletion 1p19q due to lack of economic resources. Due to neurological deterioration in the presentation and location of the tumor, 50 Gy radiotherapy was decided in 25 fractions plus concomitant temozolamide and subsequently adjuvant. In the control MRI at the end of treatment, only post-surgical changes are mentioned, the patient clinically walks, presents normal mobility in 3 of the 4 extremities, only presents left brachial paresis of 4/5. It takes 10 months of survival since the diagnosis with clear functional improvement.
Case 3
A 33-year-old male patient with 3-month history of headache and right hemiparesis 4/5, motor dysphasia associated with discrete pyramidal release, brain MRI that reports in supratentorial projection and at the level of the left temporal region, of subcortical location. Evidence of neoplastic and occupational nodular image, with a solid intra-axial appearance. It has irregular edges, but defined. No signs of perilesional edema are observed, mass effect is produced by shifting the midline to the right, from 13mm to septum pellucidum, with collapse of the left lateral ventricle. It shows heterogeneous hypointensity in sequences weighted in T1 and hyperintensity in T2. Presents content of cystic aspect, with rounded cystic areas in its interior.
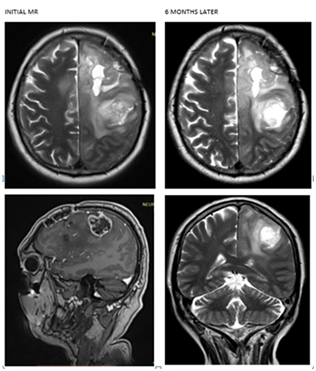
A partial resection of 60% of the tumor lesion was performed with report of WHO grade III astrocytoma pathology. Initiates Radiotherapy alone 60 Gy in 30 fractions. By control MRI with cystic aspect area showing signal hypointensity in T1 and high intensity signal in sequences weighted in T2, surrounded by important peri-lesional edema of cytotoxic aspect that affects up to the left basal para-hippocampal region. So it is decided to start adjuvant chemotherapy with temozolamide every 4 weeks for 6 cycles. Currently the patient is without neurological deficit, under anticomitant treatment and 14 months after diagnosis (Figure 2).
Case 4
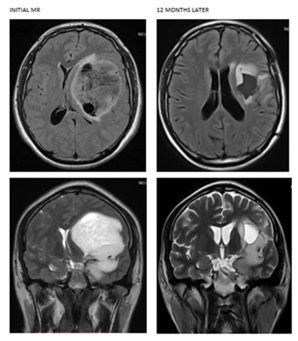
A 65-year-old male patient with motor dysphasia and right hemiplegia, brain MRI with left parietal mass, cytotoxic edema and displacement of the anterior horn of the left lateral ventricle, was taken to total resection with subsequent recurrence at 8 months being taken to partial resection of 40%, the report of pathology indicates astrocytoma grade II of the WHO. No respective mutations could be performed and the patient only accepted treatment with radiotherapy without chemotherapy, for which he received 40 Gy radiotherapy in 20 sessions, radiotherapy was not completed due to increased perilesional cytotoxic edema and worsening of symptoms. It was proposed to continue chemotherapy alone with Temozolamide; however, the patient refused treatment again. He is currently under surveillance with motor dysphasia and right hemiplegia 13 months after diagnosis of the disease (Figure 3).
Review of literature
Because primary brain tumors are a heterogeneous group of neoplasms with a variety of evolution and treatment strategies, the prognostic factors and treatment options must be carefully reviewed by a multidisciplinary group of management experts.
Pronostic and predictive factors
To guide the general treatment of tumors of the central nervous system, it is essential to know certain prognostic and predictive factors of the disease.
Factors of bad prognosis for low-grade gliomas. We have:
- Age greater than or equal to 40 years
- Tumor size ≥ 6 cm
- Tumor that crosses the middle line
- Dominant histology: Astrocytoma
- Neurological deficit at the time of presentation
With more than 2 factors, the low-grade glioma is considered HIGH RISK and should be treated aggressively because the prognosis is worse than a low-grade glioma with low risk.
On the contrary, the factors of good forecast for low grade gliomas are:
- Age under 40 years
- Absence of neurological deficit at the time of presentation.
- Complete surgical resection.
- Adequate functional status.
These factors must be taken into account to take behavior because the average survival demonstrated in high risk patients is only 3.9 years compared to 10.8 years in patients with low risk.3
Response predictors
There are several predictors of response related to overexpression of genes and genetic mutations in both glioblastomas, high grade and low grade astrocytomas, of which 3 will be mentioned:
- Delection 1p19q: The combination of the loss of chromosome 1p and 19q was identified as a univariate predictor of prolongation of overall survival in patients with pure oligodendroglioma and remains a significant predictor after adjustment for age and tumor grade variation (p <.01).4 It is present in 70 to 90% of oligodendrogliomas, in 40% of oligoastrocytomas and in only 3% of astrocytomas.5
- Methylated MGMT: Methylguanine methyl transferase (MGMT) is a DNA repair enzyme, which repairs damage to DNA produced by alkylating agents. The methylated state of the enzyme means that there is no active enzyme to repair the damage, which translates into chemosensitivity.6 The published frequency of this hypermethylation in gliomas varies widely from 35-73% in glioblastomas, being around 40% in the primary ones and up to 70% in the secondary ones, from 50-84% in the diffuse astrocytomas of degree III and 43-93% in diffuse grade II astrocytomas.7 The wide range of these results is due, in part, to the great technical challenges that this determination demands. The methods for the analysis of MGMT are many, and some require great technology and experience, which has repeatedly resulted in divergent results in different laboratories for the same tumor, as well as a great difficulty in evaluating the inter-laboratory concordance rates.
- IDH 1 mutations: The importance of the IDH gene mutation gains great prognostic relevance according to several studies. The presence of said mutation gives a more favorable prognosis.5 IDH1 mutations have been found in more than 10% of patients with glioblastoma multiforme and in 50 to 70% of oligodendrogliomas.
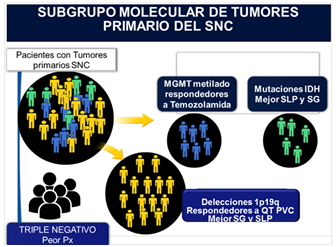
Molecular sub-group of primary CNS tumors (Table 1)
Based on the molecular presentation of the predictors of response, we can divide patients with primary tumors of the central nervous system into the following molecular subgroups to guide the systemic treatment:8,9
- MGMT methylated group: which will be good responders to Temozolamide.
- Group with HDI mutations: which will have better progression-free survival (PFS) and overall survival (OS).
- Group with deletions 1p19q: which will be responders to chemotherapy especially with PVC scheme (procarbazine, lomustine, vincristine) and will have in turn better SG and SLP.
We include in the group that we will call Triple negative those who do not have any of the mentioned mutations, being their prognosis more reserved.
Modifications in the classification of the tumors of the central nervous system
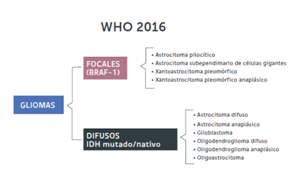
Based on the inclusion of molecular and genetic biology, the WHO has published the new classification of tumors of the central nervous system, according to the new classification the gliomas differ in low-grade focal gliomas (pilocytic astrocytomas, pleomorphic xanthoastrocytomas) when they express mutations in the BRAF-1 gene, the IDH gene being in the native genotype (not mutated). On the other hand, the group of diffuse infiltration gliomas (astrocytomas, oligodendrogliomas, oligoastrocytomas) is subdivided by subdividing them according to cell line and the expression of the HDI mutation and co-deletion 1p 19q. In other words, the new classification of tumors of the WHO SNC (2016) separates these tumors according to the expression of these genes, since they correlate with different predictions (Table 2).5
Systemic treatment in grade IV astrocytoma (glioblastoma multiforme)
According to the NCCN 2018 guidelines, currently the recommendation is the maximum surgical cytoreduction plus 60 Gy Radiotherapy concurrent with Temozolamide followed by adjuvant Temozolamide every 4 weeks for 6 cycles. In patients older than 70 years or poor ECOG, only RT or Temozolamide and in patients with absence of methylation of MGMT, only RT.10
Traditionally it was considered that QT has a marginal value in high grade gliomas. Based on Stupp's study, published in 2005, concomitant chemo-radiotherapy is currently considered the new standard of treatment in young patients with glioblastoma with good KPS and in patients with oligodendroglioma and anaplastic oligoastrocytoma.
In the phase III study, Stupp was able to demonstrate an increase in overall survival of 2.5 months, with an absolute benefit of 37% reduction in the risk of death, in addition to an increase in the PFS of 1.9 months with a statistically significant reduction in 46% risk of progression.11 Therefore, from this study, the addition of chemotherapy to radiotherapy and subsequently adjuvant chemotherapy with Temozolamide is considered the new standard of treatment in grade IV astrocytomas.
Systemic treatment in grade III asthrocytoma
According to the NCCN 2018 guidelines, currently the recommendation in patients with oligodendroglioma and anaplastic oligoastrocytoma with 1p19q deletion is maximum surgical cytoreduction plus external radiotherapy and adjuvant chemotherapy with PVC as category 1 or external radiotherapy and chemotherapy with temozolamide, or only chemotherapy with temozolamide or PVC as category 2 B. In patients with anaplastic astrocytoma and in those with oligodendroglioma and anaplasic oligoastrocytoma without 1p19q deletion, external RT is recommended as category 1, radiotherapy and chemotherapy with temozolamide or only chemotherapy with temozolamide or PVC scheme as category 2 B. patients with anaplastic gliomas with KPS less than 60 only hypofractionated radiotherapy or only chemotherapy with temozolamide or PVC is recommended as category 2 B or only better medical support.10
Until the last decade there was no clear indication of the use of chemotherapy in anaplastic gliomas, but information was available from a few uncontrolled studies showing that anaplastic gliomas (recurrent oligodendrogliomas and anaplastic oligoastrocytomas) are tumors sensitive to chemotherapy and that 60 70% of the recurrent patients respond to chemotherapy with PVC scheme (procarbazine, lomustine, vincristine), hence the hypothesis that the chemotherapy with PVC in the adjuvant context at the time of diagnosis could improve survival. That is why Van Den Vent performs the first phase III study in patients with recently diagnosed anaplasic oligodendroglioma operated on and randomizes them to adjuvant treatment with either RT alone versus RT plus concomitant chemotherapy with PVC scheme. Surprisingly, a significant increase in SG and SLP was achieved, with an absolute benefit of 12 months in SG and 11 months in SLP, with a significant reduction in the risk of death of 44%.12 In the analysis by subgroup, those with deletion 1p19q showed the best results with a survival not reached until the analysis of the study versus 112 months of the population that only received radiotherapy. Likewise, PFS is significantly greater in patients with 1p19q deletion with 157 months of PFS for those who received the combined treatment versus 50 months in the group with RT alone. Hence, the PVC scheme plus adjuvant radiotherapy is considered as the new treatment standard in anaplastic gliomas.
Benefit of temozolamide in anaplasic gliomas
Until a few years ago there was doubt about the benefit of Temozolamide alone in these patients. The phase II studies showed objective responses of 75% and complete answers of 38% with this type of chemotherapy. Thus, a phase III study called NOA-04 was conducted in which 318 patients with anaplastic gliomas (with 52.6% of anaplastic astrocytoma) were randomized to 3 arms: adjuvant RT 60 Gy in 6 weeks, adjuvant chemotherapy with scheme PVC 4 cycles or adjuvant chemotherapy with Temozolamide for 8 cycles every 4 weeks. Patients in the progressing radiotherapy group received either rescue PVC or temozolamide, and patients in both groups of progressing chemotherapy received radiotherapy. The primary objective was the time to failure of radiotherapy or chemotherapy and the secondary objectives of SLP and molecular analysis of deletion 1p19q and MGMT. It should be mentioned that about 60% had the MGMT promoter methylated and that more than 50% did not have the 1p19q deletion and about 66% had non-mutated HDI. In the study, there were no differences in time to treatment failure in all groups, nor significant differences in PFS, in general anaplastic astrocytomas showed less response to treatment. However, MGMTM methylation and IDH mutation was associated with better SLP in all treatment arms.13
The NOA study demonstrates the feasibility of starting post-operative treatment with either RT or QT in anaplastic gliomas. Methylation of the MGMT promoter, 1p19q deletion and the IDH-1 mutation emerge as predictive and predictive markers, with a better response to QT.
Systemic treatment of low grade gliomas
Surgery remains the most important therapeutic modality in this type of tumors.10 There is no consensus regarding radiotherapy treatment. In the EORTC 22845 study, immediate Radiotherapy was associated with better SLE at 5 years (44% vs 37%) but without difference in SG. That is why some oncologists differ the indication of radiotherapy until the progression of the disease.14 However, in high-risk patients with low-grade gliomas it has been shown that early radiation therapy benefits both SLP and SG. Hence the indication of early onset of RT in patients with high-risk low-grade gliomas.14
Chemotherapy is not the traditional pillar of treatment, it is considered category 2 B, however, objective responses have been shown with adjuvant Temozolamide of up to 61%, and in those patients with low grade oligodendrogliomas with deletions 1p19q, high response rates are reported (75% overall response and 38% complete answers with PVC scheme). So chemotherapy should be considered as part of the treatment.
The RTOG 9802 study is clear evidence of the benefit of chemotherapy; in the study, 251 patients with grade II WHO gliomas were randomized with supratentorial lesions and KPS> 60, who were over 40 years old with any resection or those who were younger than 40 years with subtotal resection (ie patients with high risk factors) to receive either radiotherapy alone or radiotherapy plus chemotherapy with adjuvant PVC for 6 cycles. The average OS was more than 8.5 years for the RT group plus chemotherapy versus 7.5 years for those who only received RT, SG was reported for more than 5 years in 72% of the combined treatment group versus 63% of the group that only Received RT. The SLP was significantly higher in the radiotherapy plus chemotherapy group with greater than 6.1 years vs 4.4 years with radiotherapy alone. The study concludes that QT with PVC after RT improves both the SG and the SLP in patients with low-grade gliomas with characteristics of high risk: age over 40 years with any type of resection and in children under 40 years with subtotal resection or only biopsy.
That is why in the NCCN 2018 guidelines mention that in patients with low-grade gliomas with safe maximum resection of low risk, they can be left under observation or complement with adjuvant RT, chemotherapy in them is considered category 2 B. Without However, in patients with high risk or with subtotal resection or only biopsy, category 1 is given radiotherapy plus PVC adjuvant, if a PVC scheme is not available, chemotherapy with temozolamide plus radiotherapy is category 2B.10
White therapy in tumors of the central nervous system
High-grade glioma tumors are highly vascularized due to overexpression of vascular endothelial growth factor (VEGF). Several studies have been tested with Bevacizumab, a humanized monoclonal antibody against VEGF that is active in recurrent malignant gliomas. With overall response rates of 63.6% and 31.8% complete responses and a median of SLP of 5.1 months in glioblastoma multiforme and 4.6 months in anaplastic gliomas.MED of SG 8.8 months for glioblastoma and 11.2 months for anaplastic gliomas.15,16
Discussion
Although in our country we do not have the chemotherapy agents lomustine and procarbazine, which are part of the chemotherapy scheme considered first line for low-grade gliomas with deletion 1p19q, the presentation of low-grade glioma cases treated with temozolamide (category 2 B), show an important clinical and imaging response and with survival greater than expected in the clinical context of each patient with significant disease and neurological compromise. This shows that temozolamide can be considered as an alternative for different gliomas if chemotherapy is not available in countries like ours.
Conclusion
Due to the heterogeneity of the primary tumors of the CNS, the treatment must be multidisciplinary, with adequate classification of patients in both the risk groups and in the molecular subgroups in order to receive personalized treatment. However, the primary treatment is adequate surgery. Adjuvant treatment depends on the degree and characteristics of risk as well as molecular classification. In patients with glioblastoma multiforme, 60 Gy plus adjunctive Temozolamide and adjuvant radiotherapy is category 1. In patients with oligodendroglioma and anaplastic oligoastrocytoma with 1p19q deletion, 60Gy + Adjuvant radiotherapy is considered category 1. In patients with anaplastic astrocytoma and anaplastic gliomas without 1p19q deletion only external RT as category 1 or radiotherapy plus Temozolamide or chemotherapy with adjuvant PVC scheme is category 2 B.
References
- Siegel R, Miller K, Ahmedin J. American Cancer Society: Cancer Facts and Figures 2016. Atlanta, Ga: American Cancer Society, 2016. July 11, 2016.
- CBTRUS, statistical report: primary brain tumors in the United States, 1995-1991 Chicago: central Brain Tumor registry of the United States 2002.
- Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. Eur J Clin Oncol. 2002; 20(8):2076‒2084.
- Smith JS, Perry A, Borell TJ, et al. Alterations of Chromosome Arms 1p and 19q as Predictors of Survival in Oligodendrogliomas, Astrocytomas, and Mixed Oligoastrocytomas. J Clin Oncol. 2000;18(3):636‒645.
- Facundo L, Gonzalo D. Neuropathology: Diagnosis with Molecular Biology. Revista Médica Clínica Las Condes. 2017;28(3)352‒359.
- Cankovic M, Nikiforova MN, Snuderl M, et al. The role of MGMT testing in clinical practice: A report of the Association for Molecular Pathology. J Mol Diagn. 2013;15(5):539‒555.
- Jansen M, Yip S, Louis DN. Molecular pathology in adult gliomas: diagnostic, prognostic, and predictive markers. Lancet Neurol. 2010;9(7):717‒726.
- Ahmadi R, Stockhammer F, Becker N, et al. No prognostic value of IDH1 mutations in a series of 100 WHO grade II astrocytomas. J Neurooncol.2012;109(1):15–22.
- Figarella-Branger D, Maues de Paula A, Colin C, et al. Histomolecular classification of adult difuse gliomas: the diagnostic value of inmunohistochemical markers. Rev Neurol (Paris). 2011;167(10):683‒690.
- Guias NCCN version 1. 2018.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 2005;352(10):987‒996.
- van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long term follow up of EORTC Brain tumor group Study 26951. J Clin Oncol. 2013;31(3):344‒350.
- Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874‒5880.
- Karim AB, Afra D, Cornu P, et al. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis Radiat Oncol. Int J Radiat Oncol Biol Phys. 2002;52(2):316‒324.
- Yu Z, Zhao G, Zhang Z, et al. Efficacy and safety of bevacizumab for the treatment of glioblastoma (Review). Exp Ther Med. 2016;11(2):371‒380.
- Gil MJ, de Las Peñas R, Reynés G, et al. Bevacizumab plus irinotecan in recurrent malignant glioma shows high overall survival in a multicenter retrospective pooled series of the Spanish Neuro-Oncology Research Group (GEINO). Anticancer Drugs. 2012;23(6):659‒665.