Volume : 1
Review
Medical device alternatives to restless legs syndrome (RLS) Dopaminergic Drugs
Fred Burbank,1 Cheryl Segawa2
1Salt Creek International Women’s Health Foundation, USA
2Biomedical Engineering, Sensory NeuroStimulation Inc, USA
Received: August 08, 2018 | Published: August 22, 2018
Summary
Restless Legs Syndrome (RLS) is a common neurological disorder, affecting 2.5–15% of the general Western population. For one–third to one–half of RLS suffers, daily function is impaired due to sleep loss. RLS patients dread the onset of each RLS attack because attacks are so uncomfortable. Drugs that cross the blood–brain barrier are currently the most widely accepted treatment for RLS. In the 1980s ergot and non–ergot derived dopaminergic drugs were proposed as treatments of RLS. During the five year period between 2005–2009 publications on Pub Med containing the words “restless legs” and “dopamine” reached a peak of 334 articles. However, like the appearance of articles describing tartive dyskinesia following widespread use of anti–psychotic drugs in 1970s, reports began surfacing of worrisome mid– and longer–term complication following dopaminergic drug treatment of RLS.
The most common dopaminergic drug side effect is referred to as “augmentation,” a mild–sounding word for a very troublesome phenomenon: worsening of RLS severity during the course of treatment. Worsening includes greater RLS severity than before the start of drug therapy, expansion of RLS dysphoria from the legs to the arms, and the experience of RLS symptoms earlier and earlier in the day. Concern about augmentation is now so high that an annual, half–day, postgraduate course devoted to mitigating augmentation began at the 2016 SLEEP meeting in Denver, CO.
Because initial dopaminergic drug treatment of RLS seemed so promising, medical device treatments of RLS have at times been dismissed out–of–hand. Because devices have no long–term, drug–like side effects, they need to be evaluated soberly. Diverse devices have been proposed for RLS treatment. Most postulate leg abnormalities as the etiology for RLS toward which their device is directed. Before describing RLS devices, themselves, we will provide a brief history of RLS drug therapy, a description of RLS epidemiology, and an evaluation of RLS disease modulators. A limited review of published RLS treatment devices will follow. A more detailed description of the Relaxis® (Sensory NeuroStimulation, Inc., San Clemente, CA, USA) counter–stimulation system is provided as it is the only Class II, prescription RLS device that has been cleared by the United States Food and Drug Administration (FDA) for treating RLS–related sleep disturbance.
Keywords: restless legs syndrome, medical devices, meta–analysis, clinical trials, sham effect, placebo effect
Abbreviations
FDA, food and drug administration; RLS, restless legs syndrome; WED, Willis–Ekbom disease; IRLS, international restless legs syndrome
RLS History
Restless Legs Syndrome (RLS), also known as the Willis–Ekbom Disease (WED), was described in 1672 and 1685 by the English physician Thomas Willis.1,2 He observed bizarre movements during RLS attacks and concluded that RLS caused great discomfort. However, he did not appear to understand that during an RLS attack patients choose to move about in bizarre ways to diminish RLS discomfort. The movements associated with RLS are similar to stretching to resolve a leg cramp. The discomfort of a leg cramp is relieved by voluntarily stretching the cramped muscles. Like stretching, RLS movements are volitional movements made by the RLS sufferer to diminish RLS discomfort. To treat RLS Willis prescribed tincture of opium, a then well–known pain killer. He thus set the stage for treating RLS with drugs.
In 1945, over two centuries later, Karl Axel Ekbom coined the term “restless legs syndrome” and focused attention on why RLS patients move their legs during attacks.3 In his words, “As long as the [RLS] sensations prevail, the patients are forced to keep moving their legs, which generally gives them some relief.” Without using the term, Ekbom described patient–generated counter–stimulation. Patient leg movement is a counter–stimulus to the dysphoria of RLS, one that temporarily decreases RLS dysphoria. Like Willis, Ekbom prescribed drugs to treat RLS. Interestingly, he described an informal, placebo–controlled trial comparing improvement in patients who received placebo pills with those given vasodilators. In his trial, drugs performed significantly better than the placebos, more firmly establishing RLS as a disorder to be treated with drugs.
RLS epidemiology
To date, epidemiology studies of RLS have only succeeded in measuring the burden of the disease within and between various populations and characterizing general features of RLS. Unlike epidemiology studies that connected cigarette smoking to lung cancer or a mosquito–born virus infection to yellow fever, no fundamental RLS etiology has emerged from these studies.4–7 Despite much effort, the etiology of RLS remains unknown.
RLS prevalence has been estimated to be between 2.5–15% in the general population.8,9 Across many studies, RLS prevalence appears to be a function of age, gender and, perhaps, race.
RLS prevalence is age–dependent and can start early in childhood.10 Most reports indicate that RLS prevalence increases with age,11–15 with no prevalence increase after the age of 50.12 Prevalence is reported to be over two times higher in women than in men.13–20
It is unclear how much RLS prevalence differs by race. Yeh et al surveyed 28 studies that used a uniform diagnostic criteria and found racial differences.9,21 However, in another study no racial difference was observed among RLS patients of different races living in the same city.22
RLS disease modulators
Sensory Stimuli
Rozeman et al studied the effect of external sensory stimulation during simulated RLS attacks.23 Though the study was small, they observed less RLS leg discomfort when an external sensory input (a counter–stimulus) was applied at the time of a simulated RLS attack.
Genetics
Substantial genetic heterogeneity is present within the RLS population.24 A family history of RLS in the range of 50% has been widely reported,8,10,14,15,25 with family history associated with earlier age of RLS onset10,14,15,26 and with increased RLS severity.27 Autosomal dominant genetics has been reported.28 Potential RLS genes are being pursued29–36 with 6 RLS gene candidates described.37
Diurnal, seasonal, and latitudinal effects
Other RLS modulators include diurnal,38,39 seasonal,40 and latitudinal variations41 with higher prevalence at night, in the summer, and at more northerly latitudes.
Primary versus secondary RLS
Primary RLS
In the vast majority of patients suffering from RLS, no associated disorder or illness is present to explain the presence of RLS.8,42,43 In patients with primary RLS, the anatomic site of origin appears to be in the central nervous system above the level of the spinal cord and below the level of the cerebral cortex.43 It is speculated that the site of origin may be at the subcortical level, perhaps at the level of the thalamus or the cerebellum.44
Secondary RLS
Because RLS is common, it has been observed in association with a wide variety of disorders and diseases. Some have interpreted these associations as causal links, but such causal interpretations need to be viewed with great caution. General population studies are less prone to bias and have shown a convincing association of RLS with a narrow list of factors: pregnancy, kidney disease, and iron metabolism.45 As the first step in treating secondary RLS, the underlying disease is addressed. Unlike renal disease and iron metabolism disorders, pregnancy is self resolving.19
Pregnancy
Higher RLS prevalence has been reported in pregnant women than in nulliparous women or men in the general population.19,46 In the third trimester, one–third of pregnant women have RLS.47 RLS slowly decreases in prevalence starting four weeks prior to delivery and then rapidly decreases after delivery.48
Kidney disease
The relationship between RLS and end stage renal disease was first proposed by Callaghan in 1966.49 RLS prevalence is higher in patients on chronic hemodialysis than in the general population.46,50 Patients on hemodialysis are at risks of longer or incomplete dialysis sessions because of RLS.51 Effective treatment of RLS during hemodialysis would benefit these patients.
Iron deficiency or iron metabolism abnormalities
The relationship between iron deficiency or iron metabolism abnormalities and RLS is not clear. Data indicates high RLS prevalence in patients with iron deficiency, chronic menorrhagia, and repeated blood donation.52–54 Low cytosolic iron concentrations have been observed in the brain stem tissues of RLS patients,55,56 and decreased transport of iron into the central nervous system through the blood brain barrier has been reported.57 The frequency of low serum ferritin may increase with age in RLS patients, which would parallel increased RLS prevalence with age.s
On the other hand, iron or ferritin deficiency could not be shown to contribute to RLS in a cross–sectional study of serum iron, ferritin, transferrin, and soluble transferrin receptor levels.59 Free serum iron, transferrin, and ferritin concentrations were similar in subjects with and without RLS. In this study, only soluble transferrin receptor concentration was higher in patients with RLS. A relationship between known iron–related genes with RLS and known RLS–related genes with serum iron parameters could not be established.60 Furthermore, iron–related blood tests have not been able to predict the diagnosis of RLS.61 In patients with hereditary hemochromatos is treated with phlebotomy, RLS symptoms were most likely to occur when serum ferritin fell below 25µg/L yet were observed even during times of iron overload.62 Clinical trials of RLS patients with iron supplements have led to conflicting results as well.63–66
Other risk factors
RLS has been reported to be linked with:
- peripheral neuropathy, diabetes, and Parkinson’s disease;8
- with higher rates of headache, social isolation, depression, reduced libido, hypertension, and heart problems;12
- with obesity, hypertension, loud snoring, drinking alcohol, and smoking;13
- with hypertension, arthritis, gastroesophageal reflux, depression, anxiety, diabetes, and sleep apnea;67
- and with psychiatric disorders.9
By contrast, in a large general population study, an association between hypertension, physical inactivity, obesity, diabetes, or smoking and RLS could not be established.19
Evaluating RLS treatments: placebo and treatment effect size measurements
RLS treatments are best understood in light of prospective, randomized, double–blind, parallel clinical trials or meta–analyses of such trials. Therefore, understanding control (“placebo” for drug trials and “sham” for medical device trials) and treatment effect sizes is crucial to assessing RLS treatments.
Arbitrary paper and pencil tests units
The goal of any trial is to determine if active treatment is superior to control treatment. RLS trials have used the International Restless Legs Syndrome Study Group (IRLS) scale as a measure of RLS severity68,69 and have used subjective sleep scales such as the Medical Outcomes Study Sleep Problems Index II (MOS–II) scale as a measure of sleep disturbance associated with RLS.70–72 These types of instruments are patient–reported surveys that generate outcomes scores in arbitrary units, as opposed to natural physical units such as blood pressure, height, or weight. Arbitrary units are very difficult to interpret, clinically.
The IRLS scale measures RLS severity.68,69,73 It consists of ten questions, with each question having a potential score of 0 to 4. On a scale from 0 to 40, high IRLS scores indicate greater RLS severity; low scores, less severity. Improvement on the IRLS scale is a decrease in score.
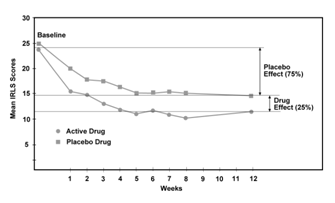
The magnitude of placebo effect on the IRLS scale may be seen by examining the time course of a typical RLS drug trial. Figure 1 presents IRLS scores over the course of a typical dopamine agonist, 12–week trial comparing placebo and treatment group severity in IRLS units.74 Placebo effect represents approximately 75% of total effect, a finding seen in many RLS drug trials. Drug therapy provides only a small, additional 25% benefit over placebo treatment. The cost of this additional benefit is adverse RLS drug events (Figure 1).75–80
|
Figure 1 Graph showing the time course of a comparison of an RLS drug with placebo over a 12-week clinical trial.74 Placebo effect accounts for approximately 75% of drug effect. |
The MOS–II scale has been shown to be reliable and valid for the assessment of sleep quality in RLS patients.70,72,81 This scale characterizes sleep quality during the prior four weeks on a scale of 0 (no problems) to 100 (very disturbed sleep). Like the IRLS scale improvement on the MOS–II scale is also a decrease in score.
Standard effect sizes
Because IRLS and subjective sleep quality scores are reported in arbitrary units, these results are more meaningfully examined as standardized effect sizes.82,83 Standardized effect sizes are computed by dividing a raw effect size in its arbitrary units (0 to 40 for the IRLS scale or 0 to 100 on the MOS–II scale) by a standard deviation measurement. Effect sizes are, then, standard deviation units. Cohen and Rosenthal characterized “small” standardized effect sizes as being between 0.2 to 0.3, medium effects centered around 0.5, “large” effects around 0.8, and “very large” effects around 1.30.82,84
Fulda and Wetter performed a meta–analysis to separately estimate the magnitudes of treatment and placebo responses in RLS trials.85 The standardized placebo effect size for the IRLS scale was very large (–1.48, 95% CI: –1.81 to –1.14). That is, the IRLS scale is very, very sensitive to placebo effect (Figure 1). For standardized subjective sleep quality measurements, placebo effect size was small (–0.27, 95% CI:–0.36 to –0.18). Subjective sleep scores are much less sensitive to placebo effect than IRLS scores. IRLS and subjective control measures will be used as benchmarks to help evaluate RLS medical device studies presented below.86
If the control arm of a trial shows little or no sham effect, blinding will be judged to have been inadequate. Since trial outcome is the difference between treatment and control, when placebo effect is artificially small because of inadequate blinding, treatment superiority over control will be falsely magnified.
RLS etiologies and treatments
Proponents of any RLS treatment believe that their therapy targets the underlying cause of RLS or at least targets a strong contributing factor. Consequently, RLS etiology and treatment are intertwined and will be discussed together. Although not all agree–especially medical device developers who believe that RLS is causes by an abnormality of the legs–RLS is generally believed to be a brain disorder.43
Imbalance of a Specific Neurotransmitter: RLS Drugs
The historical and current “gold standard” of RLS treatment is drugs. Most RLS drugs are postulated to address imbalances of specific brain neurotransmitters. Many families of neurotransmitters exist in the brain: amino acids, amines, amides, peptides, soluble gasses, endogenous opioids, and others. A recent meta–analysis of RLS drug treatments evaluated a wide range of these neurotransmitters.87 The medications examined included dopamine–related (pramipexole, ropinirole, levodopa with dopa decarboxylase inhibitor); opioid–related (methadone); amino acid–related (gabapentin enacarbil, cabergoline, gabapentin, pregabalin, carbamazepine); and amine–related (clonidine) drugs. The existence of such a wide range of drug families or drug classes to treat RLS suggests that no “silver bullet” pharmacological treatment” has yet been found.
Limited RLS drug effectiveness
When examined by standardized effect size or when examined graphically (Figure 1), RLS drug treatments are, at best, modestly effective. Ondo et al have concluded that a difference between placebo and drug in the change of IRLS score from baseline to study completion of –5 points is “…clinically meaningful.”88 Trenkwalder et al have proposed a –6 point difference as a success cut–off.89 In a large systematic review by Scholz et al examining 7,365 patients and 30 trials, dopamine agonists were superior to placebo treatment by –5.74 IRLS units, which would characterize these drug treatments as “clinically meaningful” by the criteria above.90 However, a difference of –5.74 IRLS units translates into a standardized effect size of only –0.131 (Hedges’s g statistic), placing this difference in the “small” effect size range.
In the same meta–analysis, standardized effect size for subjective sleep quality inventories showed superiority of drugs over placebo of –0.400 (Hedges’s g statistic), placing drug superiority for treating RLS–related sleep problems in the “medium” effect size range. As we will see below, a medium sized sleep improvement by RLS drugs is not substantially different than sleep improvement reported by one of the medical device treatments described below, the Relaxis system.91
Worrisome RLS drug side effects
In addition to being limited in effectiveness, RLS drugs have serious side effects including excessive drowsiness, impaired blood pressure regulation, rebound (worsening of RLS symptoms in the morning), augmentation (greater RLS severity than prior to drug therapy, earlier onset of RLS symptoms in the evening, and spreading of RLS symptoms to the arms), compulsive activities such as compulsive gambling and compulsive sexual behavior, and more.92–94 Medical devices for the treatment of RLS warrant consideration as they have no such side effects.
With this brief review of the RLS drug therapy–the gold standard of RLS treatment–as a background, we will examine published medical device RLS therapies. As discussed earlier, when available we will use sham effects size measurements as estimates of the quality of clinical trial blinding in the studies below. Small sham effect size suggests poor blinding; larger sham effect size suggests adequate blinding. Poor blinding will favor falsely reporting superiority of treatment over control.
Inadequate perfusion (systemic, legs, and feet)
Inadequate tissue perfusion in the legs has been postulated as a cause of RLS. Near–infrared light heating of the legs increases tissue perfusion in the legs. Greater improvement in IRLS scores for patients assigned to light treatment over sham treatment has been reported.95 In this study sham effect size was very large, –1.646 (Hedges’s g statistic), suggesting that patient blinding was successful.86,96,97
Enhanced external counterpulsation therapy (EECP) was developed to diminish ischemia symptoms in individuals with angina or heart failure and is similar, conceptually, to an intra–aortic balloon pump.98,99 EECP as an RLS treatment showed mixed results; initial reports of superior long–term improvement could not be repeated.100,101
Studying the effects of whole body vibration (WBV), the authors reported that RLS patients had higher resting skin blood flow than controls.102 In a follow–up, cross–over design study they treating RLS patients with WBV.103 Subjects assumed a “semi–squatting” stance on a whole–body vibrating platform with knees flexed to 40 degrees. Treatment consisted of ten, 30–second bouts of vibration three times per week for two weeks. Sham patients stood on the platform in the same “semi–squatting” position but vibration was not activated. Decreased RLS severity on the IRLS scale was observed. However, from examination of sham effect size (–0.0853, Hedges’s g statistic) it appears that blinding was incomplete. Crossover designs in RLS trials are prone to poor blinding as patients in both arms of such trials readily distinguish sham from treatment, making trial results questionable.86 The authors were aware of this study weakness stating, “…subjects were able to feel the difference” between sham and treatment.
Faulty leg veins
Sclerotherapy was used to occlude incompetent leg veins in RLS patients under the assumption that faulty veins caused or contributed to RLS symptoms. Follow–up showed RLS improvement with recurrence rates of 8% and 28% at 1 and 2years, respectively, leading the author to conclude that RLS responds to sclerotherapy.104 This study had no control subjects and was not blinded which raises concerns about its findings.
Venous and/or lymphatic stasis in the legs has been postulated to contribute to or cause RLS. In a sham–controlled trial, RLS severity decreased significantly more in the group treated with pneumatic compression stockings than in the control group.105,106 Sham effect size was 1.066 (Hedges’s g statistic) which is large suggesting that blinding was effective in this trial.86 Again, the authors were aware of blinding limitations.
Defective leg nerves
Acupuncture is thought to at least partially exert its effects through an influence on nerves. A systematic review of the effect of acupuncture on RLS symptoms concluded that insufficient evidence exists to determine whether acupuncture is an effective RLS treatment or not.107
In an open–label trial comprised of 22 women and 8 men with moderate to severe primary RLS bilateral wraps were placed around the feet to apply pressure to the abductor hallucis and flexor hallucis brevis muscles. Pressure was applied to generate afferent nerve impulses from the feet.108 The study had no control population. IRLS scores improved, but without sham control, distinguishing between therapeutic effect and placebo effect is impossible.
Dysphoric brain sensations projected to the legs during RLS attacks
While a minority of RLS patients may have vascular or nerve abnormalities in their legs, since RLS is so widespread and seen in young and older people, the legs of the vast majority of RLS sufferers are most likely with the range of normal. Furthermore, since drugs currently used for RLS treatment come from very diverse drug families or drug classes and have limited benefits, one specific RLS neurotransmitter abnormality seems difficult to accept, as well. We propose an alternative etiology.
Leg sensations in the brain
Sensory Input during Wakefulness
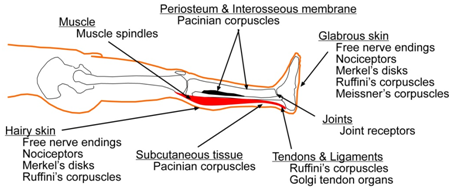
The brain “knows” about the detailed state of the legs from diverse sensory inputs originating in the legs (Figure 2). Specialized biological transducers in the legs convert changing pressure, pain, heat, cold, vibration detected at different frequencies by Pacinian corpuscles, Meissner’s corpuscles, and Merkel’s discs, fine and crude touch, static pressure, and proprioception into nerve impulses that transmit complex sensory information from the legs to the brain.
Three distinct somatic sensory neuronal pathways transmit leg sensations through the spinal cord to the thalamus, which, in turn, transmits these signals to the sensory cortex where they are mapped to specific brain locations.109
|
Figure 2 Illustration showing a leg with its major sensory transducers. The skin is shown in peach, periosteum and interosseous membrane in black, and the gastrocnemius muscle in red.109 |
Sensory input during sleep–the thalamocortical loop
When one is asleep, perception of the external world diminishes greatly. We postulate that an abnormality in the neuronal circuit that runs between the thalamus (which receives all sensory input from the legs) and the sensory cortex (which brings these sensations into consciousness) is central to the etiology of RLS. The circuit is referred to as the “thalamocortical loop.”109–111 During sleep, and to a lesser extent during times of sleepiness or drowsiness, the thalamocortical neurons enter an oscillatory state, become synchronized with nerve activity the cortex, and disconnect the cortex from the outside world. The brain is partially “unplugged” from sensations originating outside the brain. Loud sensations will awake a sleeper. Softer sensations are not perceived. If an elephant could tip toe softly through a sleeper’s bedroom, the sleeper might not perceive the elephant’s presence.
When so disconnected, the sensory cortex receives sensory input not from the legs, themselves, but from the thalamus. Because a very diverse range sensations comes from the legs to the thalamus (Figure 2), should information in the thalamocortical loop become activated and scrambled–as in an RLS attack–the scrambled signal–like the dysphoria of RLS–would be very difficult to describe.
We postulate that RLS dysphoria starts somewhere in the “thalamocortical loop” and is “mapped” or “projected” from the brain to the legs. That is, although RLS dysphoria occurs entirely within the brain, it is perceived by the sleeper or sleepy person to originate in the legs. Since no bizarre leg stimuli are present during an RLS attack, the attacks are, in effect, nightmare–like, tactile hallucination–like, or sensory seizure–like experiences. This process may be similar to the phantom limb syndrome experienced by some amputees.112 Those amputees experience distorted images of the amputated leg as if the leg were still attached to the body. Phantom limb sensations arise in the brain but are mapped to the absent limb’s prior cortical representation within the brain and are perceived to come from the external world. Consistent with this view, some amputees have reported RLS in their absent limb.113–116
Preemptive versus contemporaneous treatment
None of the RLS drug treatments or the RLS medical device treatments reviewed above is administered at the time of an RLS attack. All are all administered on fixed–schedules, unrelated to the timing of RLS attacks. In contrast, Relaxis therapy, described below, is based on the principle of counter–stimulation and is only administered at the time of an RLS attack.
Counter–stimulation theory
The principle of counter–stimulation is known from everyday life. Anyone who has wielded a hammer and hit his or her thumb has subconsciously applied its principles. Upon impact, the struck hand is withdrawn and shaken. Instead of experiencing pure pain, shaking produces a host of non–pain sensations (counter–stimuli) that enter the brain from the injured hand and diminish the perceived intensity of pain. Like hand–shaking, vibration can serve as a counter–stimulus to pain. Several biological transducers (Figure 2) respond to vibration, and vibratory stimulation has been used as a counter–stimulus for a range of pain disorders.117–127
If, as we have postulated, RLS attacks originate in the brain and are projected or mapped to the legs, how does vibration from the legs break an RLS attack? A counter–stimulus does not have reach the brain from the same neuronal pathway as the source of pain or dysphoria to be effective. The counter–stimulus just has to reach the brain. Effective counter–stimulation can reach the brain from neuronal pathways separate from the pathway of pain. For example, light and sound sensations–which enter the brain through cranial nerves–have both been demonstrated to diminish experimentally generated extremity pain which enters the brain through the spinal chord and thalamus.118,128–131 We postulate that RLS dysphoria originates in the brain, is perceived by the brain to originate in the legs, and can be disrupted by real sensory input from the legs.129–131
The concept of counter–stimulation is well–known to the RLS community. An early diagnostic criterion for RLS states, “Motor restlessness–patients move to relieve the discomfort, for example walking, or to provide a counter–stimulus to relieve the discomfort, for example, rubbing the legs”.69,132
The rapidity with which most RLS attack can be broken by standing and walking about suggests that breaking an RLS attack may be similar to the evaporation of a dream or nightmare upon awakening. One minute a dreamer might be chased by a tiger, but upon awakening, the tiger ceases to exist. Just as awakening erases a dream by presenting real–world stimuli to the dreamer, standing and walking presents real sensory inputs from the legs and terminates most RLS attacks. The flood of real sensory inputs caused by standing and walking quickly dispels the nightmare–like somatic sensations of RLS. Standing and walking, however, are incompatible with sleep. Relaxis was developed to present a device–generated counter–stimulus to RLS dysphoria, allowing a patient to break an RLS attack without having to become fully awake, get out of bed, and pace about the house.
The Relaxis system
The Relaxis system consists of a controller and a pad that provide vibration as a counter–stimulus to RLS dysphoria at the time of an attack. When activated, the Relaxis pad vibrates at an intensity chosen by the patient. After 30 minutes of counter–stimulation the vibration ramps down slowly and then gently stops so a sleeping patient is not awakened by a sudden change in vibration intensity. A photograph of a Relaxis pad and its controller is shown in Figure 3.

|
Figure 3 Photograph of a Relaxis pad placed under a patient’s legs and lower thighs. Patient is shown adjusting the controller to her vibration comfort level. |
Comparisons to shams
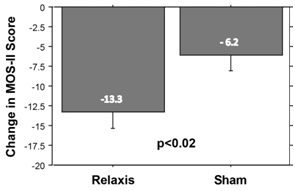
Two parallel, sham–controlled, randomized, prospective, multi–center clinical trials have evaluated the effect of vibratory counter–stimulation treatment on RLS–related sleep disturbance.133 In these two trials of 158 patients with moderately severe, primary RLS, sleep quality was measured with the MOS–II inventory. Sham pads were physically indistinguishable from vibrating pads. During an RLS attack patients assigned vibrating pads could focus attention on vibration whose intensity each patient controlled. Patients assigned to sham sound or sham light pads could focus attention on light or sound, tuned to an intensity that they felt was comforting. Improvement in the MOS–II sleep inventory from baseline was significantly greater in patients receiving a Relaxis pad than those receiving a sham pad (Figure 4). Furthermore, placebo effect size and other measurements made in the two Relaxis clinical trials indicated good trial blinding.86
|
Figure 4 Bar graph showing 115% greater improvement in MOS-II scores for patients assigned a Relaxis pad compared with those assigned a sham pad. Error bars are +/- 1 SEM. |
Comparison to FDA–approved RLS drugs
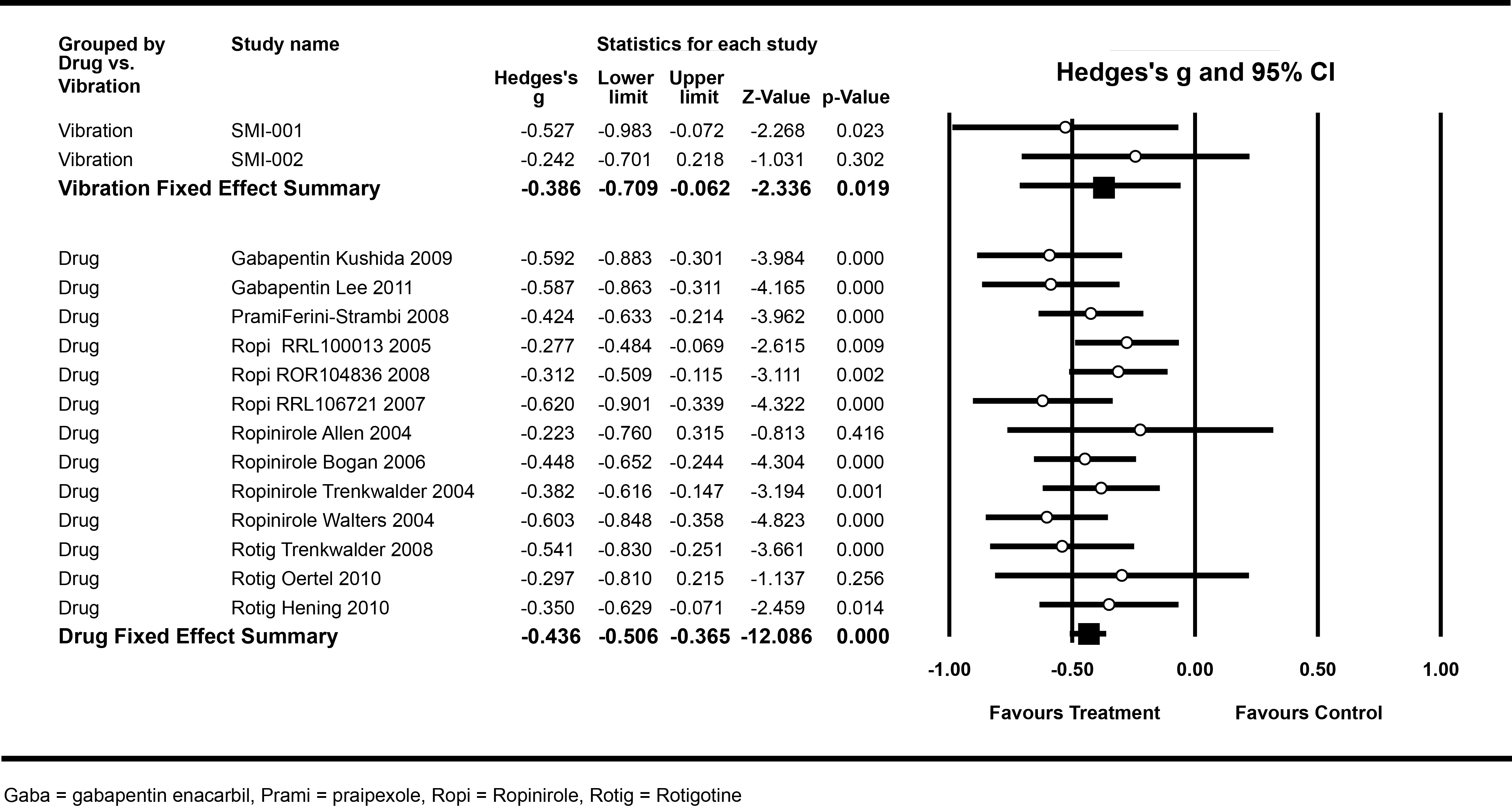
A meta–analysis comparing the safety and efficacy of Relaxis with US Food and Drug Administration (FDA)–approved RLS drugs–the current RLS treatment gold standard–was conducted (Figure 5).91 No significant difference was observed in sleep improvement scores between patients treated with vibratory stimulation (–0.386) and those receiving an approved RLS drug (–0.436, P>0.70). In this meta–analysis sham pads and placebo pills performed comparably.86
Stand alone or RLS drug therapy adjuvant
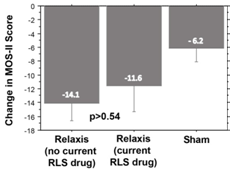
In the two vibration trials,133 study patients were either on a stable FDA–approved drug dosage (58patients) or not taking any RLS drugs (100patients). No significant difference in sleep improvement was observed between the no–drug and stable–drug groups (Figure 6). It appears that Relaxis can be used as an adjuvant for patients on RLS medication or as a stand–alone RLS treatment.
|
Figure 5 Meta-analysis Forest plot of Hedges’s g statistics for effect sizes for individual trials and for grouped vibratory stimulation and drug trials. |
|
Figure 6 Bar graph showing no significant difference in MOS-II sleep improvement scores between patients using Relaxis pads, whether or not they were receiving an RLS drug. Error bars are +/- 1 SEM. |
Adverse events in the vibration trials133
In the two Relaxis trials, nine patients assigned a Relaxis pad and one patient assigned a sham pad reported that during RLS attacks, use of the assigned pad made RLS dysphoria worse. This is best understood knowing that some sensory inputs are disturbing to some people and not to others. For example, the scraping sound of fingernails on a blackboard gives some people the “willies” while others pay little attention to the sound. Similarly, approximately 10% of RLS patients dislike the sensation of vibration. For these patients, use of a vibrating pad during an RLS attack exacerbated the attack because they experience two sensations they hate–RLS dysphoria and vibration. Simply stopping Relaxis use resolved RLS symptom worsening in all 9 patients.
Other reported adverse events–also mild to moderate in severity–included leg cramping, tingling, soreness, pain, and motion sickness. After discontinuing device use, all adverse events resolved without intervention. No long–term side effects were observed.
A possible alternative to RLS drugs for selected RLS patients
Relaxis has been shown to provide greater sleep improvement than sham pads (Figure 4) and is comparable in sleep improvement to many RLS drugs (Figure 5). Reported side effects were few, mild to moderate in severity, and all resolved upon discontinuing device use. Consequently, selected RLS patients who may benefit from Relaxis are:
- Newly diagnosed patients who do not want to start RLS drugs
- Patients on RLS drugs who want to decrease their dosage or stop their RLS drug or drugs altogether
- Patients on RLS drugs with intolerable drug side effects
- Patients on RLS drugs who need additional help with sleep and do not want to increase their drug dosage or add another RLS drug
Conclusion
RLS is a common sleep disorder whose current treatment gold standard is drugs. When viewed graphically or as standardized effect sizes, RLS drug benefits are limited while drug side effects may be substantial. Many medical device treatments for RLS have been proposed: leg heating to increase perfusion, whole body vibration to increase blood flow, enhanced external counterpulsation to increase cardiac output, venous sclerotherapy to treat incompetent veins, pneumatic compression stockings to diminish venous and/or lymphatic stasis, and acupuncture and foot compression to modify nerve impulses in the legs. Although some of these treatments have shown potential, none have been cleared by the FDA as a Class II medial device.
The Relaxis system is an FDA–cleared prescription device. It consists of a vibrating pad and its digital controller. Two clinical trials have demonstrated that the Relaxis system provides significant sleep improvement with the degree of sleep improvement comparable to improvement seen in RLS drug trials. Selected RLS patients may benefit from the Relaxis system.
Acknowledgment and Disclosures
Fred Burbank, M.D. is the primary inventor of the Relaxis system, the Chief Executive Officer and Board Chairman of Sensory NeuroStimulation, Inc., the manufacturer of the Relaxis system, and he owns Sensory NeuroStimulation, Inc. stock. Contact: FBurbank@cox.net. Cheryl Segawa, M.S. is a paid consultant of Sensory NeuroStimulation, Inc., and she owns stock options in the company.
References
- Willis T. De anima brutorum. Wells and Scott; 1672.
- Willis T. Anonymous Instructions for curing the Watching evil in The London practice of physick. London: Bassett and Crooke; 1685. p. 402–407.
- Ekbom KA. Restless legs: a clinical study. Acta Med Scand. 1945;158 (Supplement):1–222, 1945.
- Burbank F. U.S. lung cancer death rates begin to rise proportionately more rapidly for females than for males: a dose–response effect? J Chronic Dis. 1972;25(8):473–9.
- Findlay. John Williams and the early history of yellow fever. Br Med J. 1948;2(4574):474–476.
- Toner JM. The distribution and natural history of yellow fever as it has occurred at different times in the United States. Public Health Pap Rep. 1873;1:359–384.2.
- Reed W, Agramonte A. Landmark article. Feb 16, 1901: The etiology of yellow fever. An additional note. By Walter Reed, Jas. Carroll and Aristides Agramonte. JAMA. 1983;250(5):649–658.
- Zucconi M, Ferini–Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med. 2004;5(3):293–299.
- Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012;16(4):987–1007.
- Picchietti D, Allen RP, Walters AS, et al. Restless legs syndrome: prevalence and impact in children and adolescents––the Peds REST study. Pediatrics. 2007;120(2):253–266.
- Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137–2141.
- Ulfberg J, Nystrom B, Carter N, et al. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16(6):1159–1163.
- Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2012;53(1):547–554.
- Tison F, Crochard A, Leger D, et al. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology. 2005;65(2):239–246.
- Rijsman R, Neven AK, Graffelman W, et al. Epidemiology of restless legs in The Netherlands. Eur J Neurol. 2004;11(9):607–611.
- Rothdach AJ, Trenkwalder C, Haberstock J, et al. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology. 2000;54(5):1064–1068.
- Bjorvatn B, Leissner L, Ulfberg J, et al. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med. 2005;6(4):307–312.
- Kim J, Choi C, Shin K, et al. Prevalence of restless legs syndrome and associated factors in the Korean adult population: the Korean health and genome study. Psychiatry Clin Neurosci. 2005;59(3):350–353.
- Hogl B, Kiechl S, Willeit J, et al. Restless legs syndrome: a community–based study of prevalence, severity, and risk factors. Neurology. 2005;64(11):1920–1924.
- Ziaei J, Saadatnia M. Epidemiology of familial and sporadic restless legs syndrome in Iran. Arch Iran Med. 2006;9(1):65–67.
- Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119.
- Lee HB, Hening WA, Allen RP, et al. Race and restless legs syndrome symptoms in an adult community sample in east Baltimore. Sleep Med. 2006;7(8):642–645.
- Rozeman AD, Ottolini T, Grootendorst DC, et al. Effect of sensory stimuli on restless legs syndrome: a randomized crossover study. J Clin Sleep Med. 2014;10(8):893–896.
- Ondo WG, Vuong KD, Wang Q. Restless legs syndrome in monozygotic twins: clinical correlates. Neurology. 2000;55(9):1404–1406.
- Winkelmann J. Genetics of restless legs syndrome. Curr Neurol Neurosci Rep. 2008; 8(3):211–216.
- Winkelmann J, Wetter TC, Collado–Seidel V, et al. Clinical characteristics and frequency of the hereditary restless legs syndrome in a population of 300 patients. Sleep. 2000;23(5):597–602.
- Hanson M, Honour M, Singleton A, et al. Analysis of familial and sporadic restless legs syndrome in age of onset, gender, and severity features. J Neurol. 2004;251(11):1398–1401.
- Walters AS, Picchietti DL, Ehrenberg BL, et al. Restless legs syndrome in childhood and adolescence. Pediatr Neurol. 1994;11(3):241–245.
- Winkelmann J, Lichtner P, Schormair B, et al. Variants in the neuronal nitric oxide synthase (nNOS, NOS1) gene are associated with restless legs syndrome. Mov Disord. 2008;23(3):350–358.
- Winkelmann J, Schormair B, Lichtner P, et al. Genome–wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39(8):1000–1006.
- Schulte EC, Kousi M, Tan PL, et al. Targeted resequencing and systematic in vivo functional testing identifies rare variants in MEIS1 as significant contributors to restless legs syndrome. Am J Hum Genet. 2014;95(1):85–95.
- Spieler D, Kaffe M, Knauf F, et al. Restless legs syndrome–associated intronic common variant in Meis1 alters enhancer function in the developing telencephalon. Genome Res. 2014;24(4):592–603.
- Winkelmann J, Czamara D, Schormair B, et al. Genome–wide association study identifies novel restless legs syndrome susceptibility loci on 2p14 and 16q12.1. PLoS Genet. 2011;7(7):e1002171.
- Schormair B, Plag J, Kaffe M, et al. MEIS1 and BTBD9: genetic association with restless leg syndrome in end stage renal disease. J Med Genet. 2011;48(7):462–466.
- Kemlink D, Plazzi G, Vetrugno R, et al. Suggestive evidence for linkage for restless legs syndrome on chromosome 19p13. Neurogenetics. 2008;9(2):75–82.
- Kemlink D, Polo O, Montagna P, et al. Family–based association study of the restless legs syndrome loci 2 and 3 in a European population. Mov Disord. 2007;22(2):207–212.
- Freeman AA, Rye DB. The molecular basis of restless legs syndrome. Curr Opin Neurobiol. 2013;23(5):895–900.
- Trenkwalder C, Hening WA, Walters AS, et al. Circadian rhythm of periodic limb movements and sensory symptoms of restless legs syndrome. Mov Disord. 1999;14(1):102–110.
- Sharifian A, Firoozeh M, Pouryaghoub G, et al. Restless Legs Syndrome in shift workers: A cross sectional study on male assembly workers. J Circadian Rhythms. 2009;7:12.
- Ingram DG, Plante DT. Seasonal trends in restless legs symptomatology: evidence from Internet search query data. Sleep Med. 2013;14(12):1364–1368.
- Koo BB. Restless legs syndrome: relationship between prevalence and latitude. Sleep Breath. 2012;16(4):1237–1245.
- Evidente VG, Adler CH. How to help patients with restless legs syndrome. Discerning the indescribable and relaxing the restless. Postgrad Med. 1999;105(3):59–61, 65–6, 73–4.
- Allen RP. Controversies and challenges in defining the etiology and pathophysiology of restless legs syndrome. Am J Med. 2007;120(1 Suppl 1):S13–21.
- Allen RP, Earley CJ. Restless legs syndrome: a review of clinical and pathophysiologic features. J Clin Neurophysiol. 2001;18(2):128–147.
- Berger K, Luedemann J, Trenkwalder C, et al. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164(2):196–202.
- Garcia–Borreguero D, Egatz R, Winkelmann J, et al. Epidemiology of restless legs syndrome: the current status. Sleep Med Rev. 2006;10(3):153–167.
- Neau JP, Marion P, Mathis S, et al. Restless legs syndrome and pregnancy: follow–up of pregnant women before and after delivery. Eur Neurol. 2010;64(10):361–366.
- Goodman JD, Brodie C, Ayida GA. Restless leg syndrome in pregnancy. BMJ. 1988;297(6656):1101–1102.
- Callaghan N. Restless legs syndrome in uremic neuropathy. Neurology. 1966;168(4):359–361.
- Holley JL, Nespor S, Rault R. Characterizing sleep disorders in chronic hemodialysis patients. ASAIO Trans. 1991;37(3):M456–457.
- Beladi–Mousavi SS, Jafarizade M, Shayanpour S, et al. Restless legs syndrome: associated risk factors in hemodialysis patients. Nephrourol Mon. 2015;7(6):e31967.
- Rangarajan S, D'Souza GA. Restless legs syndrome in Indian patients having iron deficiency anemia in a tertiary care hospital. Sleep Med. 2007;8(3):247–251.
- Ulfberg J, Nystrom B. Restless legs syndrome in blood donors. Sleep Med. 2004;5(2):115–118.
- Silber MH, Richardson JW. Multiple blood donations associated with iron deficiency in patients with restless legs syndrome. Mayo Clin Proc. 2003;78(1):52–54.
- Snyder AM, Wang X, Patton SM, et al. Mitochondrial ferritin in the substantia nigra in restless legs syndrome. J Neuropathol Exp Neurol. 1999;68(11):1193–1199.
- Snyder AM, Connor JR. Iron, the substantia nigra and related neurological disorders. Biochim Biophys Acta. 2009;1790(7):606–614.
- Mizuno S, Mihara T, Miyaoka T, et al. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14(1):43–47.
- O'Keeffe ST. Secondary causes of restless legs syndrome in older people. Age Ageing. 2005;34(4):349–352.
- Berger K, von Eckardstein A, Trenkwalder C, et al. Iron metabolism and the risk of restless legs syndrome in an elderly general population––the MEMO–Study. J Neurol. 2002;249(9):1195–1199.
- Oexle K, Schormair B, Ried JS, et al. Dilution of candidates: the case of iron–related genes in restless legs syndrome. Eur J Hum Genet. 2013;21(4):410–414.
- Allen RP, Auerbach S, Bahrain H, et al. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol. 2013;88(4):261–264.
- Shaughnessy P, Lee J, O'Keeffe ST. Restless legs syndrome in patients with hereditary hemochromatosis. Neurology. 2005;64(12):2158.
- Wang J, O'Reilly B, Venkataraman R, et al. Efficacy of oral iron in patients with restless legs syndrome and a low–normal ferritin: A randomized, double–blind, placebo–controlled study. Sleep Med. 2009;10(9):973–975.
- Allen RP, Adler CH, Du W, et al. Clinical efficacy and safety of IV ferric carboxymaltose (FCM) treatment of RLS: a multi–centred, placebo–controlled preliminary clinical trial. Sleep Med. 2011;12(9):906–913.
- Grote L, Leissner L, Hedner J, et al. A randomized, double–blind, placebo controlled, multi–center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord. 2009;24(10):1445–1552.
- Earley CJ, Horska A, Mohamed MA, et al. A randomized, double–blind, placebo–controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009;10(2):206–211.
- Phillips B, Hening W, Britz P, et al. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129(1):76–80.
- Abetz L, Arbuckle R, Allen RP, et al. The reliability, validity and responsiveness of the International Restless Legs Syndrome Study Group rating scale and subscales in a clinical–trial setting. Sleep Med. 2006;7(4):340–349.
- Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–132.
- Allen RP, Kosinski M, Hill–Zabala CE, et al. Psychometric evaluation and tests of validity of the Medical Outcomes Study 12–item Sleep Scale (MOS sleep). Sleep Med. 2009;10(5):531–539.
- Hays RD, Stewart AL. Sleep measures. In: in Stewart AL, et al. editors. Measuring functioning and well–being; The medical outcomes study approach. 4th ed. Durham and London: Duke University Press; 1998. p. 235–259.
- Hays RD, Martin SA, Sesti AM, et al. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005;6(1):41–44.
- Allen RP, Kushida CA, Atkinson MJ. Factor analysis of the International Restless Legs Syndrome Study Group's scale for restless legs severity. Sleep Med. 2003;4(2):133–135.
- Walters AS, Ondo WG, Dreykluft T, et al. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: a 12–week, double–blind, randomized, parallel–group, placebo–controlled study. Mov Disord. 2004;19(12):1414–1423.
- Signorelli MS, Battaglia E, Costanzo MC, et al. Pramipexole induced psychosis in a patient with restless legs syndrome. BMJ Case Rep. 2013;2013.
- Nirenberg MJ. Dopamine agonist withdrawal syndrome: implications for patient care. Drugs Aging. 2013;30(8):587–592.
- Hornyak M, Trenkwalder C, Kohnen R, et al. Efficacy and safety of dopamine agonists in restless legs syndrome. Sleep Med. 2012;13(3):228–236.
- Voon V, Schoerling A, Wenzel S, et al. Frequency of impulse control behaviours associated with dopaminergic therapy in restless legs syndrome. BMC Neurol. 2011;11:117.
- Jones HB, George S. 'You never told me I would turn into a gambler': a first person account of dopamine agonist––induced gambling addiction in a patient with restless legs syndrome. BMJ Case Rep. 2011;2011.
- Earley CJ, Silber MH. Restless legs syndrome: understanding its consequences and the need for better treatment. Sleep Med. 2010;11(9):807–815.
- Abetz L, Arbuckle R, Allen RP, The reliability, validity and responsiveness of the restless legs syndrome quality of life questionnaire (RLSQoL) in a trial population. Health Qual Life Outcomes. 2005;3:79.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. p. 1–159.
- Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107–128.
- Rosenthal JA. Qualitative descriptors of strength of association and effect size. Journal of Social Service Research. 1996;21(4):37–59.
- Fulda S, Wetter TC. Where dopamine meets opioids: a meta–analysis of the placebo effect in restless legs syndrome treatment studies. Brain. 2008;131(pt 4):902–917.
- Burbank F. Sleep improvement for restless legs syndrome patients. Part IV: meta–analysis comparison of effect sizes of vibratory stimulation sham pads and placebo pills. Journal of Parkinsonism and Restless Legs Syndrome. 2014;4:35–40.
- Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults––an update for 2012: practice parameters with an evidence–based systematic review and meta–analyses: an American Academy of Sleep medicine clinical practice guideline. Sleep. 2012;35(8):1039–62.
- Ondo W, Roth T, Grieger F, et al. Minimal clincially important improvement in IRLS total score in patients with restless legs syndrome: A post hoc analysis from a 6–month placebo–controlled US–based study of rotigotine transdermal system. Neurology. 2016;80:0592013.
- Trenkwalder C, Kohnen R, Allen RP, et al. Clinical trials in restless legs syndrome––recommendations of the European RLS Study Group (EURLSSG). Mov Disord. 2007;22 Suppl 18:S495–504.
- Scholz H, Trenkwalder C, Kohnen R, et al. Dopamine agonists for restless legs syndrome. Cochrane Database Syst Rev. 2011;(3):CD006009.
- Burbank F, Buchfuhrer MJ, Kopjar B. Improving sleep for patients with restless legs syndrome. Part II. Meta–analysis of vibration therapy and drugs approved by the FDA for treatment of restless legs syndrome. Journal of Parkinsonism and Restless Legs Syndrome. 2013;3:11–22.
- GlaxoSmithKline staff. Horizant product Insert. 2011.
- GlaxoSmithKline staff. Requip product insert. 2011.
- Boehringer Ingelheim staff. Mirapex product Insert. 2011.
- Mitchell UH, Myrer JW, Johnson AW, et al. Restless legs syndrome and near–infrared light: An alternative treatment option. Physiother Theory Pract. 2011;27(5):345–351.
- Mitchell UH, Johnson AW, Myrer B. Comparison of two infrared devices in their effectiveness in reducing symptoms associated with RLS. Physiother Theory Pract. 2011;27(5):352–359..
- Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS ONE. 2010;5(12):e15591.
- Zheng ZS, Yu LQ, Cai SR, et al. New sequential external counterpulsation for the treatment of acute myocardial infarction. Artif Organs. 1984;8(4):470–477.
- Arora RR, Chou TM, Jain D, et al. The multicenter study of enhanced external counterpulsation (MUST–EECP): effect of EECP on exercise–induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33(7):1833–1840.
- Rajaram SS, Shanahan J, Ash C, et al. Enhanced external counter pulsation (EECP) as a novel treatment for restless legs syndrome (RLS): a preliminary test of the vascular neurologic hypothesis for RLS. Sleep Med. 2005;6(2):101–106.
- Rajaram SS, Rudzinskiy P, Walters AS. Enhanced external counter pulsation (EECP) for restless legs syndrome (RLS): preliminary negative results in a parallel double–blind study. Sleep Med. 2006;7(4):390–391.
- Mitchell UH, Johnson PK. Vibration and skin blood flow changes in subjects with restless legs syndrome. JPRLS. 2014;4:9–16.
- Mitchell UH, Hilton SC, Hunsaker E, et al. Decreased symptoms without augmented skin blood flow in subjects with RLS/WED after vibration treatment. J Clin Sleep Med. 2016;12(7):947–952.
- Kanter AH. The effect of sclerotherapy on restless legs syndrome. Dermatol Surg. 1995;21(4):328–332.
- Eliasson AH, Lettieri CJ. Sequential compression devices for treatment of restless legs syndrome. Medicine (Baltimore). 2007;86(6):317–323.
- Lettieri CJ, Eliasson AH. Pneumatic compression devices are an effective therapy for restless legs syndrome: a prospective, randomized, double–blinded, sham–controlled trial. Chest. 2009;135(1):74–80.
- Cui Y, Wang Y, Liu Z. Acupuncture for restless legs syndrome. Cochrane Database Syst Rev. 2008;CD006457.
- Sullivan J, Olson D. RESTIFFIC, a unique pressure foot wrap, is more effective than a dopamine agonist in reducting the symtome of moderate to severe restless legs syndrome. Neurology. 2015;84 (14 Supplement):2962015.
- Purves D, Augustine GJ, Fitzpatrick D, et al. Neuroscience: Fourth Edition. Sunderland, MA: Sinauer Associates, Inc; 2008.
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215.
- McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol. 1994;4(4):550–556.
- Giummarra MJ, Georgiou–Karistianis N, Nicholls ME, et al. Corporeal awareness and proprioceptive sense of the phantom. Br J Psychol. 2010;101(pt 4):791–808.
- Giummarra MJ, Bradshaw JL. The phantom of the night: restless legs syndrome in amputees. Med Hypotheses. 2010;74(6):968–972.
- Skidmore FM, Drago V, Foster PS, et al. Bilateral restless legs affecting a phantom limb, treated with dopamine agonists. J Neurol Neurosurg Psychiatry. 2009;80(5):569–570.
- Vetrugno R, Alessandria M, D'Angelo R, et al. "Phantom" restless legs syndrome. J Neurol Neurosurg Psychiatry. 2010;81:122–123.
- Estivill E, de la Fuente–Panell V, Segarra–Isern F, et al. [Restless legs syndrome in a patient with amputation of both legs]. Rev Neurol. 2004;39(6):536–538.
- Ward L, Wright E, McMahon SB. A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain. 1996;64(1):129–138.
- Longe SE, Wise R, Bantick S, et al. Counter–stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport. 2001;12(9):2021–2025.
- Inui K, Tsuji T, Kakigi R. Temporal analysis of cortical mechanisms for pain relief by tactile stimuli in humans. Cereb Cortex. 2006;16(3):355–365.
- Wall PD, Cronly–Dillon JR. Pain, itch, and vibration. Arch Neurol. 1960;2(4):365–375.
- Lundeberg T, Abrahamsson P, Bondesson L, et al. Effect of vibratory stimulation on experimental and clinical pain. Scand J Rehabil Med. 1988;20(4):149–159.
- Lundeberg T. A comparative study of the pain alleviating effect of vibratory stimulation, transcutaneous electrical nerve stimulation, electroacupuncture and placebo. Am J Chin Med. 1984;12(1–4):72–79.
- Aminabadi NA, Farahani RM, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9(6):33–40.
- Blitz B, Dinnerstein AJ, Lowenthal M. Attenuation of experimental pain by tactile stimulation effect of vibration at different levels of noxious stimulus intensity. Percept Mot Skills. 1964;19:311–316.
- Paice JA, Shott S, Oldenburg FP, et al. Efficacy of a vibratory stimulus for the relief of HIV–associated neuropathic pain. Pain. 2000;84(2-3):291–296.
- Kakigi R, Shibasaki H. Mechanisms of pain relief by vibration and movement. J Neurol Neurosurg Psychiatry. 1992;55(4):282–286.
- Lundeberg T. Naloxone does not reverse the pain–reducing effect of vibratory stimulation. Acta Anaesthesiol Scand. 1985;29:212–216.
- Gardner WJ, Licklider JCR, Weisz AZ. Suppression of pain by sound. Science. 1960;132(3418):32–33.
- Riley JF, Levine FM. Counterstimulation and pain perception: effects of electrocutaneous vs. auditory stimulation upon cold pressor pain. Pain. 1988;35(3):259–264.
- Melzack R, Weisz AZ, Sprague LT. Stratagems for controlling pain: Contributions of auditory stimulation and suggestion. Exp Neurol. 1963;8(3):239–247.
- Morosko TE, Simmons FF. The effect of audio–analgesia on pain threshold and pain tolerance. J Dent Res. 1966;45(6):1608–1617.
- The International Restless Legs Syndrome Study Group Restless Legs Syndrome Rating Scale; Instructions for authors. National Heart, Lung, and Blood Institute; 2014.
- Burbank F, Buchfuhrer MJ, Kopjar B. Sleep improvement for restless legs syndrome patients. Part I: Pooled analysis of two prospective, double–blind, sham–controlled, multi–center, randomized clinical studies of the effects of vibrating pads on RSL symptoms. Journal of Parkinsonism and Restless Legs Syndrome. 2013;3:1–10.