Volume : 1
Research
Protein S100B: is there a correlation with clinical neurological background in pediatric patients with congenital heart disease?
FLuis Antonio Pando-Orellana,1,2 Juan Calderón-Colmenero,2 Nancy Lucero Martínez–Rodríguez,2 Leonardo Del Valle-Mondragón,3 Víctor Manuel Espinoza-Gutiérrez,2 Jorge Luis Cervantes-Salazar,2 Juan Verdejo-Paris,2 Alfonso Buendía-Hernández,2 Armando Vega-López,1 Pedro José Curi-Curi2
1Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, México
2Departamento de Cardiología Pediátrica, Instituto Nacional de Cardiología, México
3Departamento de Farmacología, Instituto Nacional de Cardiología “Ignacio Chávez”, México
Received: July 07, 2018 | Published: August 28, 2018
Abstract
Objective: S100Β protein has been proposed as a brain injury biomarker in several clinical scenarios. We aimed to determine whether a correlation exists between S100Β serum levels and clinical background variables at the pre-operative period of pediatric patients with congenital heart disease.
Methods: A prospective case-control study was designed including paediatric patients from one month to with congenital heart disease admitted for surgical treatment during a 3-month period. We studied 44 patients at the pre-operative period and divided them in two groups: 20 with clinical neurological background and 24 without them. Clinical pediatric neurological background variables were obtained, and serum levels of S100Β protein were measured using the ELISA “sandwich” technique.
Results: The cut-off for S100Β serum level in patients with clinical neurological background variables was 16 pg/ml, with sensibility and specificity values of 70% and 70.8%, respectively. S100Β protein levels greater than 16pg/ ml correlated with clinical neurological background variables (p=0.014, OR=2.556, and 95% CI=1.205-5.418). Neurological clinical background variables before operation may modify operative resilience and the risk of neurological complications.
Keywords: Biomarkers, neurological variables, congenital heart disease
Introduction
Congenital heart diseases are the most frequent malformations in pediatric population, with a prevalence of 6 to 8 cases per 1000 live births.1 Advances in diagnosis and surgical techniques allowed many cases to reach adult life, but the risk of central nervous system damage continues to be one of the most feared morbidities in cardiovascular surgery.2 A great variety of neurodevelopmental disorders have been identified in up to 50% of pediatric patients, which unfortunately cannot be predicted at the pre-operative period. These disorders include weight, height and cognition deficits, that are usually detected at the postoperative period of the patients with grown-up corrected congenital heart disease.
The first diagnosis approach for neurological disorders is made by means of a clinical neurologic examination, and must be completed with neurophysiological tests and neurological imaging such as computed tomography, PET and SPECT.3,4 These studies cannot be carried out immediately in the newborn or infant period because of hemodynamic instability, critical condition of patients, unavailability or increased costs. Besides these factors, the studies cannot always lead to quantify neurological damage in order to predict clinical outcome. A biomarker is therefore needed not only to detect brain damage, but also to predict the clinical neurologic outcome. The S100B protein, fully detected in the brain, is produced by astrocytes in physiological conditions and different clinical scenarios such as cranial trauma, cerebral ischemia, neurodegenerative disorders, chronic inflammatory cerebral disease, cardiac arrest, and cardiopulmonary bypass.5–19
The aim of this study was to find out if there is any correlation between S100B protein serum concentration levels and clinical neurologic background at the pre-operative period of pediatric patients with congenital heart disease.
Methods
We designed a prospective case-control study that included all pediatric patients (one month to 18years old), with congenital heart disease, admitted at our institution for surgical treatment, in a 3month period of time. The only exclusion criteria was previous cardiac surgery. A clinical neurological background was obtained by means of a clinical history that was applied to the main responsible parent, relative or closest person in charge of the pediatric patient.
We emphasized our interest in neurological peri-natal and post-natal antecedents. Peri-natal neurologic signs considered as positive were fetal suffering (Neonatal asphyxia), pre-eclampsia, eclampsia, and APGAR score <8 during the first minute and/or at the 5minutes after birth. Post-natal neurological background considered as positive were: neurodevelopmental deficit, epilepsies, syndromatic phenotype and/or genotype, and clinical neurological sign focalization. Definitions of these terms were obtained from the respective clinical practice councils.20,21 We considered fetal suffering as positive major criteria for peri-natal neurologic antecedents, and clinical neurologic pathological signs for post-natal neurologic antecedents. All the remaining factors were considered as positive minor criteria for peri-natal and post-natal clinical neurological background. Demographical and anthropometric complementary data such as gender, weight, height, congenital heart disease type, and pre-operative oxygen saturation was also registered. Table 1 Patients were divided in two groups:
- With clinical neurological background and
- Without clinical neurological background.
Variable |
With clinical neurological background |
Without clinical neurological background |
P |
||
|---|---|---|---|---|---|
n(%)/Mean±SD |
n(%)/Mean±SD |
||||
Age |
6±4.812(1-16) |
6.54±4.89(1-15) |
0.714 |
||
Gender |
Female |
11(55%) |
12(50%) |
0.771 |
|
Male |
9(45%) |
12(50%) |
|||
Weight |
17.64±12.25(3 - 51) |
23.75±17.78(5 - 60) |
0.201 |
||
Height |
103.63±31.64(55-162) |
110.63±31.87(64 -160) |
0.471 |
||
Peri-natal |
Fetal distress |
8(40%) |
|||
Pre-eclampsia |
4(20%) |
||||
Eclampsia |
1(5%) |
||||
APGAR |
5 |
1(5%) |
|||
6 |
1(5%) |
||||
7 |
2(10%) |
||||
8 |
8(40%) |
6(25%) |
0.418 |
||
9 |
3(15%) |
||||
APGAR |
7 |
1(5%) |
|||
8 |
4(20%) |
||||
9 |
8(40%) |
6(25%) |
0.312 |
||
10 |
2(10%) |
||||
Neuro- |
Deficient |
14(70%) |
15(62.5%) |
0.752 |
|
development |
Normal |
6(30%) |
9(37.5%) |
||
Post-natal |
Epilepsia |
1(5%) |
|||
background |
Syndromatic antecedents |
4(20%) |
4(16.7%) |
1 |
|
Clinical neurologic compromise |
7(35%) |
1(4.2%) |
0.015 |
||
Congenital disease type |
Non cianotic |
12(60%) |
15(62.5%) |
1 |
|
Cianotic |
8(40%) |
9(37.5%) |
|||
Preoperative oxygen saturation (%) |
84.95±11.27(63-98) |
87.17±10.35(63-98) |
0.501 |
||
Table 1 Population characteristics
In order to include pediatric patients within the group with clinical neurological background we required a positive major peri-natal criteria, plus a positive major post-natal clinical alteration or; at least two positive perinatal and/or post-natal minor clinical pathological criteria. This study was approved by our institution´s ethics committee, and signed consent was provided for every case enrolled.
Determination of protein S100B serum concentration levels
Peripheral blood samples were obtained from all patients at the pre-operative period and centrifuged at 3000rpm for 15minutes at room temperature. Sample plasma aliquots were obtained and frozen at -80°C until analysis. S100B serum levels were measured by an ELISA technique (S100B [Human] ELISA KIT, ABNOVA; KA0037) in two incubation periods for a total period of 120minutes. During the first incubation period, a monoclonal specific antibody was added (biotinylated anti S100B antibody) for 60minutes. Afterwards, it was added to HRP -streptavidine. After 30minutes of incubation and washing, this was permitted to react with the substrate solution. The reaction was stopped by addition of an acid solution and the absorbance of the resulting product was measured. Results were Table 1. Obtained using a standard curve of S100B in accordance with manufacturer’s directions and was expressed as µg/L.
Statistical analysis
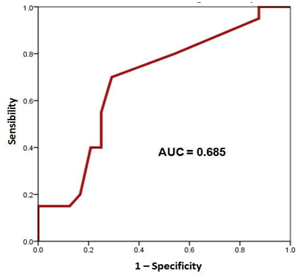
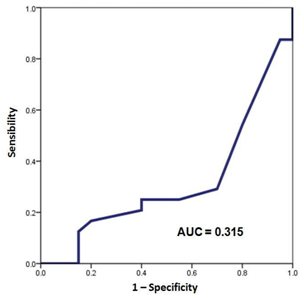
All the data was registered in a checklist at the pre-operative period. Information was stored in an electronic Excel page and processed with an SPSS statistical software v21.0 (SPSS Inc., Chicago, Ill, USA). Quantitative variables are presented as a mean, and variability ranges (minimum and maximum). Categorical data are presented by means of frequency and percentages in relation to the population at risk. S100B protein serum concentration levels were plotted by means of a ROC curve for both groups of patients and area under the curve (AUC) was determined to compare them Figure 1, Figure 2. Comparison of categorical variables between patients with clinical neurological background and the ones without it was done by means of a Fisher´s exact test. Odds ratio was also calculated with a 95% confidence interval (CI). For comparing quantitative variables that were normally distributed we performed a t Student test. Values of p<0.05 were considered as statistically significant.
Results
We included 44patients for the final analysis, divided in two groups: 20 of them with clinical neurological background, and 24without them. The demographic and clinical characteristics are shown Table 1. The group with clinical neurological antecedents had a significant difference in the presence of clinical neurological data (35%, n=7, p=0.015), compared with the group without clinical neurological antecedents. Besides this, the rest of variables did not show statistical differences, which lead to an appropriate comparability between groups.
S100B protein serum concentration levels were plotted by means of ROC curves to find the cutoff value of this protein in the presence or absence of clinical neurological background (Figure 1) (Figure 2). The area under the curve (AUC) was 0.685 for the group with clinical neurological antecedents and of 0.315 for the group without clinical neurological antecedents. The cutoff serum level for S100B to identify patients with clinical neurological background was 16pg/ml, with a sensibility and specificity value of 70% and 70.8%, respectively.
Correlation between S100B protein serum level and clinical neurological antecedents are presented in Table 2. High S100B protein serum concentration level >16pg/ml correlates significantly with the presence of clinical neurological background (p=0.014) with a 2.556 odds ratio (OR) and 95% confidence interval of 1.205 to 5.418.
S100B serum concentration level(pg/ml) |
With clinical neurological background n(%) |
Without clinical neurological background n(%) |
p |
OR (CI 95%) |
|---|---|---|---|---|
High(>16pg/ml) |
14(70%) |
7(29.2%) |
0.014 |
2.556 (1.205-5.418) |
Low(≤16pg/ml) |
6(30%) |
17(70.8%) |
Table 2 Correlation between S100B protein serum concentration levels and clinical neurological background
Discussion
Clinical anamnesis in order to obtain neurological background is difficult in pediatric population with congenital heart disease for several reasons. Because of young age, physicians must ask for this information to parents, relatives or closest persons in charge of the patient. This is a problem that cardiologists and physicians in general must deal with, particularly in developing countries, because of the low social, economic and cultural development of their populations. In addition we must also consider transcultural means of clinical proceedings while questioning.
Therefore, a biomarker that correlates with neurological clinical antecedents would be very helpful in this context, considering that surgical treatment is not to be delayed for obvious reasons of natural history of congenital heart diseases. On the other hand, the risk is increased (neurologically) if we find any neurological antecedents which on daily basis are not properly detected at the preoperative period.
In other instances we observed that the behavior of Hypoxic patients, with cyanosis have better reactions to acute hypoxic events than the ones that have normal levels of saturation of oxygen. This gave way to a protocol published recently.22
S100B protein appears to be the potential biomarker that we are looking for establishing neurological morbidity risk at the preoperative period of surgery for congenital heart disease, due to its correlation within clinical neurological antecedents, as shown in this study. This protein was discovered by Moore in 1965 when he isolated it from a sub-cellular fraction of bovine brain22–25 and was named S100 because its components are soluble in a 100% saturated ammonium sulfate at neutral pH. This protein belongs to a 25member family (E-F hand proteins calcium binding) with various configurations: alpha or beta units. The subunit β-β (S100B) is highly specific in the brain, glial cells and Schwann cells. Subunits α-β are present in the glial system, and subunits α-α were found in striated muscle, heart and kidneys.26–30 Synthesis of S100B depends on glial cells, mainly astrocytes, which regulate synaptic plasticity.31,32 Most of the protein acts intracytoplasmatically, regulating Ca++, transcription and axonal growth. In the extracellular space, S100B interacts with Receptor for Advanced Glycation End products (RAGE) receptors elevating IL-6, IL-8 and glutamate. This protein has growth factor properties.6,32
There are literature reports which evidence that S100B protein is considered as a possible biomarker for brain damage.33,34 Even though in the current study we did not measure protein S100B serum concentration levels during cardiac surgery and at the post-operative period, but there are some contamination factors during
and after surgery related to its elevation.35,39 such as anesthetics, medication, dopamine, reanimation procedures, and Cardiopulmonary bypass timing, with hypothermia during surgery, and so are cardiac shock, seizures, and sepsis during the postoperative period.
It is also important to consider that S100B levels also can also be increased, with transfusions. Therefore, it is difficult to explain the real reason that raises up serum concentration levels of protein S100B at the time of surgery and postoperatively.40–50
Neurological morbidity outcomes at this time requires not only the use of a biomarker, but also of anatomical and functional tests (e.g. PET/CT; computed tomography, electroencephalography, etc.) in order to demonstrate brain damage. This is the reason because we focused our attention before surgery, instead of the operative and postoperative periods. In addition, it is at the pre-operative period the ideal moment to use a biomarker, because it provides valuable information that complements –or eventually replaces somehow- clinical neurological antecedents in order to establish neurological morbidity risk in a pediatric patient with cardiac heart disease that is to be submitted to Cardio-pulmonary bypass.
Pre-operative mechanisms of brain damage in pediatric patients with congenital heart disease are complex, multifactorial, and not well known yet. Release of this protein may occur at the pre-natal period as a consequence of brain malformations, or because of hemodynamic alterations and metabolic issues during fetal life. At the post-natal period, S100B release may due to chronic hypoxia, brain vascular regulation disorders, brain hypoxia or brain ischemia secondary to hemodynamic instability or embolic events.51,52
Complex metabolical context in Nitrogen reactive species within anatomical substrate (astrocytes) induces its release. S100B protein increased serum concentration levels can be considered a potential reliable biomarker at low cost.
The high costs as well as low budget pending over population demanding for highly and costly specialized medical services (as well as imaging) particularly MRI and PET/CT; that correlates with clinical neurological background at the pre-operative period of pediatric patients with congenital heart disease, in which the medical priority is their heart- life threatening condition, generates a demand for such markers.
Therefore, it can be useful to identify patients with a variable status of brain damage before operation, which may probably increase their postoperative risk of neurological complications.
However, this issue should be addressed in a multicenter scale study in order to confirm these statements.
Author contributions
Luis Antonio Pando-Orellana, MD. PhD,: Concept/Design, Data analysis/interpretation, Drafting article, Critical revision of the article, Approval of article, Statistics, Overall- Data collection, Funding secured by, Responsible researcher
Juan Calderón-Colmenero, MD: Data analysis/interpretation, Drafting article, Critical revision of the article, Approval of article, overall-Data collection
Nancy Lucero Martínez–Rodríguez, PhD: Data analysis/interpretation, Drafting article, Critical revision of the article, Approval of article, Statistics, laboratory Data collection
Leonardo Del Valle-Mondragón, PhD: Data analysis/interpretation, Critical revision of the article, Approval of article, laboratory Data collection
Víctor Manuel Espinoza-Gutiérrez, MD: Data analysis/interpretation, Critical revision of the article, Approval of article, Data collection
Jorge Luis Cervantes-Salazar, MD: Critical revision of the article, Approval of article, Data collection
Juan Verdejo-Paris, MD: Critical revision of the article, Approval of article. Approval of the article.
Alfonso Buendía-Hernández, MD: Critical revision of the article, Approval of article, Data collection
Armando Vega-López, PhD: Data analysis/interpretation, drafting article, Critical revision of the article, Approval of article, Overall-Data collection
Pedro José Curi-Curi, MSc: Concept/Design, Data analysis/interpretation, Drafting article, Critical revision of the article, Approval of article, Statistics, overall-Data collection.
Acknowledgements
None.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship or publication of this manuscript.
Disclosure of grants or other funding
The authors received no financial support for the research, authorship or publication of this manuscript.
References
- Calderón-Colmenero J. Introducción. En: Attie F, Calderón-Colmenero J, Zabal C, Buendía A. Cardiología Pediátrica. México: Editorial Medica Panamericana; 2013. p. 3‒17.
- Abdul-Khaliq H, Blasig IE, Baur MO, et al. Release of the cerebral protein S-100 into blood after reperfusion during cardiac operations in infants: is there a relation to oxygen radical-induced lipid peroxidation. J Thorac Cardiovasc Surg. 1999;117(5):1027‒1028.
- Markowitz SD, Ichord RN, Wernovsky G, et al. Surrogate markers for neurological outcome in children after deep hypotermic circulatory arrest. Semin Cardiothorac Vasc Anesth. 2007;11(1):59‒65.
- Miller SF, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928‒1938.
- Ali MS, Harmer M, Vaughan R. Serum S100 protein as a marker of cerebral damage during cardiac surgery. Br J Anesth. 2000;85(2):287‒298.
- Aloe L. Rita Levi-Montalcini: The discovery of nerve growth factor and modern neurobiology. Trends Cell Biol. 2014;14(7):395‒399.
- Calderón-Colmenero J, Cervantes-Salazar JL, Curi-Curi PJ, et al. Problemática de las cardiopatías congénitas en México. Propuesta de regionalización. Arch Cardiol Mex. 2010;80(2):133‒140.
- Carrillo-González NJ, Ortuño-Sahagún D, Gudiño-Cabrera C. Identificación de isoformas del mRNA del GFAP (Proteína Fibrilar Glial Acídica) en distintas regiones del sistema nervioso de la rata adulta. En: Avances en la investigación Científica en el CUCBA. Guadalajara, México: U. de G; 2005. p. 240‒245.
- Charpentier TH, Thompson LE, Liriano MA, et al. The effects of Cap Z peptide (TRTK-12) binding to S100B-Ca2+ as examined by NMR and X-ray crystallography. J Mol Biol. 2010;396(5):1227‒1243.
- Claus W, Heizmann, Beat W, et al. The family of S100 cell signaling proteins. In: Ralph A, Bradshaw, editors. Handbook of Cell Signaling. Oxford UK: Academic Press; 2009. p. 983‒994.
- Curi-Curi PJ, Cervantes-Salazar J, Calderón-Colmenero J, et al. Resultados inmediatos en cirugía cardiovascular neonatal. Rev Invest Clin. 2012;64(2):199‒206.
- Blomquist S, Johnsson P, Lührs C, et al. The appearance of S-100 protein in serum during and immediately after cardiopulmonary bypass surgery: a possible marker for cerebral injury. J Cardiothorac Vasc Anesth. 1997;11(6):699‒703.
- Breuer AC, Franco I, Marzewski D, et al. Left ventricular thrombi seen by ventriculography are a significant risk factor for stroke in open-heart surgery. Ann Neurol. 1981;10:103‒104.
- Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450(3):191‒231.
- Drohat AC, Nenortas E, Beckett D, et al. Oligomerization state of S100B at nanomolar concentration determined by large-zone analytical gel filtration chromatography. Protein Sci. 1997;6(7):1577‒1582.
- Villarreal A, Seoane R, González Torres A, et.al. S100B protein activates a RAGE-dependent autocrine loop in astrocytes: implications for its role in the propagation of reactive gliosis. J Neurochem. 2014;131(2):190‒205.
- Angelo MF, Aguirre A, Avilés Reyes RX, et al. The proinflammatory RAGE/NF-κB pathway is involved in neuronal damage and reactive gliosis in a model of sleep apnea by intermittent hypoxia. PLoS One. 2014;9(9):e107901.
- Almaraz AC, Bobrow BJ, Wingerchuk DM, et al. Serum neuron specific enolase to predict neurological outcome after cardiopulmonary resuscitation: a critically appraised topic. Neurologist. 2009;15(1):44‒48.
- Shinozaki K, Oda S, Sadahiro T, et al. S-100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: a systematic review. Crit Care 2009;13(4):R121.
- Gonzalez FI, Miller SP. Does Perinatal asphyxia impair cognitive function without cerebral palsy? Arch Dis Child Fetal Neonatal Edition. 2006;91(6):F454‒F4559.
- Pando-Orellana L, Buendía-Hernandez A, Calderon-Colmenero JE, et al. La importancia del binomio corazón cerebro en el manejo integral de lascardiopatías congénitas. Arch Cardiología Mex. 2010;80(4):249‒254.
- 22. Pando-Orellana LA. Calderon-Colmenero J, Martinez L, et al. Protein S100B a potential biomarker of brain damage in pediatric congenital heart disease? 2018.
- Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19(6):739‒744.
- Aurell A, Rosengren LE, Karlsson B, et al. Determination of S-100 and glial fibrillary acidic protein concentrations in cerebrospinal fluid after brain infarction. Stroke. 1991;22(10):1254‒1258.
- Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-Hand type with intracellular and extracellular roles. Int J Biochem Cell Biol. 2001;3(7):637‒668.
- Marshak DR, Pesce SA, Stanley LC, et al. Increased S100 beta neurotrophic activity in Alzheimer´s disease temporal lobe. Neurobiol Aging. 1992;13(1):1‒7.
- Usui A, Kato K, Abe T, et al. S-100ao protein in blood and urine during open-heart surgery. Clin Chem. 1989;35(9):1942‒1944.
- Vaage J, Anderson R. Biochemical markers of neurologic injury in cardiac surgery: the rise and fall of S100B. J Thorac Cardiovasc Surg. 2001;122(5):853‒855.
- Zimmer DB, Cornwall EH, Landar A, et al. The S100 protein family: history, function, and expression. Brain Res Bull. 1995;37(4):417‒429.
- Selinfruend R, Barger SW, Pledger WJ, et al. Neurotrophic protein S100B stimulates glial cell proliferation. Proc Natl Acad Sci USA. 1991;88(9):3554‒3558.
- Fanó G, Mariggió MA, Angelella P, et al. The S-100 protein causes an increase of intracellular calcium and death of PC12 cells. Neuroscience. 1993;53(4):919‒925.
- Haglid KG, Yang Q, Hamberger A, et al. S-100β stimulates neurite outgrowth in the rat sciatic nerve grafted with acellular muscle transplants. Brain Res. 1997;753(2):196‒201.
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, et al. Radial glia rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101(50):17528‒17532.
- Yardan T, Erenler AK, Baydin A, et al. Usefulness of S100B protein in neurological disorders. J Pak Med Assoc. 2011;61(3):276‒281.
- Kleindienst A, Hesse F, Bullock MR, et al. The neurotrophic protein S100B: value as a marker of brain damage and possible therapeutic implications. Prog Brain Res. 2007;161:317‒325.
- Apak R, Güçlü K, Ozyürek M, et al. Cupric ion reducing antioxidant capacity assay for antioxidants in human serum and for hydroxyl radical scavengers. Methods Mol Biol. 2010;594:215‒239.
- Sakimura K, Kushiya E, Ogura A, et al. Upstream and intron regulatory regions for expression of the rat neuron-specific enolase gene. Brain Res Mol Brain Res 1995;28(1):19‒28.
- Villarreal A, Aviles Reyes RX, Angelo MF, et al. S100B alters neuronal survival and dendrite extension via RAGE-mediated NF-κB signaling. J Neurochem. 2011;117(2):321‒332.
- Zhang W, Potrovita I, Tarabin V, et al. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2005;25(1):30‒40.
- Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart disease. J Thorac Cardiovasc Surg. 2009;137(3):529‒537.
- Rosén H, Rosengren L, Herlitz J, et al. Increased serum levels of the S-100 protein are associated with hypoxic brain damage after cardiac arrest. Stroke. 1998;29(2):473‒477.
- Routsi C, Stamataki E, Nanas S, et al. Increased levels of serum S100B protein in critically ill patients without brain injury. Shock. 2006;26(1):20‒24.
- Gazzolo D, Vinesi P, Geloso MC, et al. S100 blood concentrations in children subjected to cardiopulmonary by-pass. Clin Chem. 1988;44(5):1058‒1060.
- Sanchez-Peña P, Pereira AR, Souror NA, et al. S100B as an additional prognostic marker in subarachnoid aneurysmal hemorrhage. Crit Care Med. 2008;36(8):2267‒2273.
- Fillipidis AS, Papadopoulos DC, Kapsalaki EZ, et al. Role of the S100B serum biomarker in the treatment of children distress from mild traumatic brain injury. Neurosurg Focus. 2010;29(5):E2.
- Ingebrigtsen T, Rommer B, Kongstad P, et al. Increased serum concentrations of protein S-100 after minor head injury: a biochemical serum marker with prognostic value. J Neurol Neurosurg Psychiatry. 1995;59(1):103‒104.
- Kilminster S, Treasure T, McMillan T, et al. Neuropsychological change and S-100 protein release in 130 unselected patients undergoing cardiac surgery. Stroke. 1999;30(9):1869‒1874.
- Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun. 2004;322(4):1111‒1122.
- Multicenter trial of hemodilution in ischemic stroke: background and study protocol. Scandinavian Stroke Group. Stroke. 1985;16(5):885‒890.
- Waterloo K, Ingebrigtsen T, Rommer B. Neuropsychological function in patients with increased serum levels of protein S-100 after minor head injury. Acta Neurochir. 1997;139(1):26‒32.
- Mori T, Asano T, Town T. Targeting S100B in Cerebral ischemia and in Alzheimer´s disease. Cardiovasc Psychiatry Neurol. 2010;2010:687067.
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50(5);553‒562.
- Fujita Y, Kuchimaru T, Kadonosono T, et al. In vivo imaging of brain ischemia using an oxygen-dependent degradative fusion protein probe. PLoS One. 2012;7(10):e48051.