Volume : 1
Case Report
Pleomorphic xanthoastrocytoma (PXA) vs. giant cell glioblastoma (gcGBM): a diagnostic challenge for pathologists
Argyriou S,1 Moutafidou S,1 Rigas G,1 Pazarli E,2 Efstratiou,2 Kanakis D3
1Faculty of Medicine, Democritus University of Thrace, Alexandroupolis, Greece
2Laboratory of Pathology, Papageorgiou General Hospital, Thessaloniki, Greece
3University of Nicosia Medical School, Nicosia, Cyprus
Received: August 03, 2018 | Published: September 04, 2018
Abstract
Pleomorphic xanthoastrocytoma(PXA) and giant cell glioblastoma(gcGBM) both belong to the astrocytic tumors of the central nervous system, with different grade of malignancy; the former is grade II and the latter is grade IV according to the current WHO Classification of the CNS Tumors. Due to several overlapping histopathological features between the aforementioned neoplasm sand in cases where an exact diagnosis is challenging, specific Immunohistochemical markers can be utilized. These markers in combination with the clinical and imaging findings can significantly help in the establishment of the final diagnosis and thus, in the more efficient treatment approach.
Herein, we present such a case of a frontotemporal-localized tumor and deal with the difficulty, from a pathologist’s point of view, in differentiating between these two entities, solely by the routine H&E stain. Finally, we emphasize the importance of the Immunohistochemistry and the necessity of the clinical and imaging data in concluding to the most appropriate and possible diagnosis.
Keywords: pleomorphic xanthoastrocytoma (PXA), giant cell glioblastoma (gcGBM), p53, CD34, synaptophysin, BRAFV600, MGMT
Abbreviations
PXA, pleomorphic xanthoastrocytoma; gcGBM, giant cell glioblastoma; H&E, hematoxylin and eosin
Case presentation
We report here the case of a 33-year-old woman, who was hospitalized for severe headaches of rapid onset, which were not responding to analgesic drugs. Furthermore, the patient complained of disorders of the visual acuity. Her previous medical history was devoid of any neurological symptoms. During her hospitalization, an MRI of the brain was performed, which showed a lesion within the left front temporal region with displacement of both ipsilateralanterior and middle cerebral arteries. The tumor showed no connection to the overlying meanings. Subsequently, a craniotomy was carried out, with full excision of the tumor.
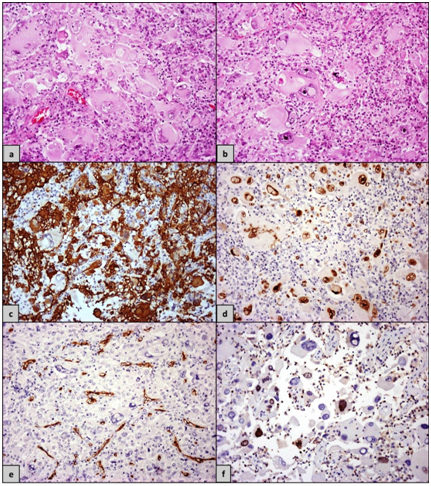
In the pathology lab, we received for frozen section two specimens of 0.5cm each. Afterwards, ten more samples of 0.7cm (maximal diameter) arrived, which were fixed in formaldehyde and in a second step embedded in paraffin. The cut surface of all the described specimens showed macroscopically alternating yellow and white areas. In the prepared frozen sections, a primary diagnosis of a malignant neoplasm, more consistent with a high-grade glioma,was made. The histopathological examination of the formaldehyde fixed and paraffin embedded sections revealed in the Hematoxylin and Eosin (H&E) stain a population of neoplastic giant cellsintermingled with smaller ones (Figures 1A) (Figure 1B). The former had several nuclei, some of which showed also the presence of nuclear inclusions. The cytoplasm of these cells appearedmarkedly eosinophilic and contained a numberof cytoplasmic inclusions. In addition, inflammatory cell infiltrates, consisting mainly by lymphocytes and lesser plasma cells were noticed among the neoplastic cells. However, mitoses were infrequent and necrosis was not identifiable in any of the tissue specimens.
The performed Immunohistochemistry showed GFAP immunoexpressionin most of the neoplastic cells, whereas a small number of the giant cells were negative for this marker (Figure 1C). Strong nuclear positivity for p53 (Figure 1D) was evident in the majority of the neoplastic cells throughout the tumor. On the contrary, the immunohistochemicalstains for CD34 (Figure 1E; the stained vessels represent internal positive control) and Synaptophysin were negative. Finally, the Ki-67/MiB-1 proliferation index reached an average of 10% and focally even15% (Figure 1F).
Discussion
The giant cell glioblastoma (gcGBM) is considered a rare variant of glioblastoma (GBM), accounting for less than 1% of all glioblastomas with the tumor beingmore common in pediatric populations. Compared to the ordinary GBM, the giant cell variant has a wider age range (affecting younger individuals), is better circumscribed, and it has a slightly better prognosis than the ordinary form of GBM.1 Macroscopically the tumor has appeared to arise from deep structures of the cerebral hemispheres though it can rarely arise from other structures of the CNS like the cerebellum.2 PXA, on the other hand, is found more superficially, involving the meninges and cerebrum (meningocerebral) and it is often accompanied by a cyst, with the occasional formation of a mural nodule within the cyst wall. Microscopically, the most striking feature of gcGBM is the presence of abundant, partially bizarre looking neoplastic giant cells, many of which are multinucleated. Often, these giant cells are angulated and may contain prominent nucleoli and cytoplasm inclusions. The tumor is further characterized by the formation of a reticulinstroma and by atypical mitoses along with per vascular lymphocytic infiltrates. Lastly, both palisading and large ischemic necroses are observed1 A molecular genetic analysis of 19 gcGBMs carried out by Meyer-Puttlitz et al.,3 showed high incidence of TP53 mutations whereas EGFR and CDK4 gene amplification was seen in only one tumor and homozygous deletion of CDKN2A was not identified at all.3 The microscopic picture of PXA shows high degree of pleomorphism with spindled cells intermingled with mono- or multinucleated giant astrocytes. The characteristic “xanthomatous” cells are multinucleated cells with intracellular accumulation of lipids. Eosinophilic granular bodies and focal collections of lymphocytes with plasma cells are also frequent as well as the presence of reticulinfibres.4 In order for a PXA to be classified as anaplastic PXA (WHO grade III), a mitotic index of >5 mitoses in 10 HPF must be present.5,6 However, the significance of necrosis in the absence of high mitotic rate is unknown.6 The molecular profile of the pleomorphic xanthoastrocytomashows high percentage of BRAFV600 mutations. Specifically, this was true for 60% (12 out of 20) of the PXAs in an analysis carried out by Martinez-Dias et al., whereas the corresponding percentage for gcGBM was only 11.1% (one out of nine).7 Similar results were found in the study of Lohkamp et al.,8 in which half (50%) of the 20 involved PXAs showed BRAFV600 mutations but no one (0%) of the 34gcGBMs. However, herein MGMT promoter hypermethylation was more common in the gcGBMs (14 out of 34; 41.2%) compared to the PXAs (3 out of 20; 15%).8 As it has been already mentioned above, there is a high degree of association between p53 and gcGBM.3 Nevertheless, it has been shown that no such relation exists between p53 and PXA according to the results of a study, where again a strong p53 immunoreactivity was observed in many tumor cells in 5 of 8gcGBMs, while the staining was negative or partially positive in 6 of 8 involved PXAs.9 On the other hand, the expression of both neuronal markers10 and CD3411 is frequently seen in PXAs.
In our case, the combination of the aforementioned histomorphologicaland Immunohistochemical features (strong p53 positivity, Ki-67/MiB-1 index of 10% [average] and negative staining for Synaptophysin and CD34), along with the clinical (short-term history, rapid growth of the tumor) and imaging (tumor’s localization far from the meanings) findings, favored the diagnosis of a giant cell glioblastoma (WHO grade IV) over that of a PXA(WHO grade II) or even an anaplastic PXA (WHO grade III). The molecular characterization of the tumor and in particular the investigation for BRAFV600 mutations and/or MGMT promoter hypermethylation would provide more information and contribute substantially in the achievement and justification of the correct diagnosis. However, this was not possible in the current case.
Undoubtedly, there is a significant level of overlap among the histomorphological findings of gcGBM and PXA; the most important of them being the multinucleated giant cells, the abundant reticulinstroma and the presence of lymphocytes, all these can indeed confuse a pathologist and cause difficulty in concluding to a definitive diagnosis, just by the use of routine H&E stain. Therefore, the current case highlights the importance of a multidisciplinary approach, where all the available data (i.e. clinical, imaging, pathological [histo-and immunohistochemistry]) must be taken into consideration, in order to come to a final diagnosis.
Acknowledgements
None.
Conflicts of interest
The author declares no conflict of interest.
References
- Ohgaki H, Kleihues P, Pate KH, et al. Giant cell glioblastoma. In Louis DN, et al. editors. WHO Classification of Tumors of the Central Nervous System Lyon. IARCP; 2016. p. 46–47.
- Queiroz LS, Faria AV, Zanardi VA, et al. Lipidized giant–cell glioblastoma of cerebellum. Clin Neuropathol. 2005;24(6):262–266.
- Meyer–Puttlitz B, Hayashi Y, Waha A, et al. Molecular genetic analysis of giant cell glioblastomas. Am J Pathol. 1997;151(3):853–857.
- Giannini C, Paulus W, Louis DN, et al. Pleomorphic xanthoastrocytoma. In Louis DN, et al. editors. WHO Classification of Tumors of the Central Nervous System. Lyon: IARCP; 2016. p. 94–97.
- Giannini C, Scheithauer BW, Burger PC, et al. Pleomorphic xanthoastrocytoma: what do we really know about it? Cancer. 1999;85(9):2033–2045.
- Ida CM, Rodriguez FJ, Burger PC, et al. Pleomorphic xanthoastrocytoma: natural history and long–term follow–up. Brain Pathol. 2015;25(5):575–586.
- Dias–Santagata D, Lam Q, Vernovsky K, et al. BRAFV600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One. 2011;6(3):e17948.
- Lohkamp LN, Schinz M, Gehlhaar C, et al. MGMT promoter methylation and BRAFV600E mutations are helpful markers to discriminate pleomorphic xanthoastrocytoma from giant cell glioblastoma. PLoS One. 2016;11(6):e0156422.
- Martinez–Diaz H, Kleinschmidt–DeMasters BK, Powell SZ, et al. Giant cell glioblastoma and pleomorphic xanthoastrocytoma show different immunohistochemical profiles for neuronal antigens and p53 but share reactivity for class III β–Tubulin. Arch Pathol Lab Med. 2003;127(9):1187–1191.
- Giannini C, Scheithauer BW, Lopes MB, et al. Immunophenotype of pleomorphic xanthoastrocytoma. Am J Surg Pathol. 2002;26(4):479–485.
- Reifenberger G, KaulichK, Wiestler OD, et al. Expression of the CD34 antigen in pleomorphicxanthoastrocytomas. Acta Neuropathol. 2003;105(4):358–364.