Volume : 1
Case Report
Primary cerebellar glioblastoma in a patient with neurofibromatosis type 1 - case report in military central hospital, Mexico Citys
Ramírez Aragón S,1 Viera Dórame Raúl Fernando,2 Moreno Estrada V,3 Mora Almanza Xochitl Guadalupe,4 Arroyo Mayorga G,5 Bravo Valle J6
1Neurocirugía, Hospital Militar Regional de Especialidades de Monterrey, México
2Neurocirugía, Hospital Militar Regional de Especialidades de Guadalajara, México
3Neurocirugia del Hospital Central Militar, Ciudad de México, México
4Neurocirugia, Hospital Militar Regional de Especialidades de Guadalajara, Guadalajara, México
5Neurocirugia del Hospital Central Militar, Ciudad de México, México
6Neurorradiologia del Hospital Central Militar, Ciudad de México, México
Received: August 28, 2018 | Published: September 27, 2018
Abstract
Glioblastoma multiforme(GBM) is common in the cerebral hemispheres, most of them may be secondary to anaplastic astrocytomas, however, GBM in the cerebellum is a rare finding. We present the only case reported in our hospital of cerebellar glioblastoma in a 75 years old patient, the former was removed subtotally. We reviewed the literature, the association between NFT1 and high-grade gliomas, aspects of Neuroimaging, as well as histopathological findings.
Keywords: glioblastoma, cerebellar, primary glioma, neurofibromatosis, posterior fossa glioma
Introduction
Glioblastoma represents 15-20% of all intracranial tumors and approximately 50% of all gliomas in adults,1–3 the annual incidence of malignant gliomas is 3-5/100,000 with a slight predominance in the male sex. Malignant gliomas can develop at all ages with peak incidence in the fifth and sixth decade of life.4,5 These tumors are located more frequently in the cerebral hemispheres, basal ganglia, thalamus, corpus callosum, and they infiltrate the white matter and/or in the depth of the gray matter. They develop secondary to a diffuse an aplastic astrocytoma, however they can arise from primary form6 and rarely they can appear in the posterior fossa.1,7 It was confirmed by clinical history, physical data in the patient and literature that was revised the association between Neurofibromatosis type 1 and the risk of developing high-grade gliomas,8 which is why we will present the present case of cerebellar glioblastoma of the posterior fossa in an adult.
Case report
A 75-year-old male patient presented at our hospital with decreased alertness, drowsiness, nausea, vomitingandoral intolerance of 1month of evolution, adding up inability to walk for a week of evolution. In patient’s history there is Neurofibromatosis type 1 what is the base diagnosis for this, diagnosed at the age of 21 years and a radical prostatectomy for the treatment of prostate cancer in September 2013.
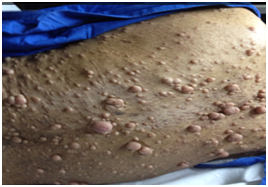
On physical examination, the patient appeared drowsy, dehydrated and was unable to walk. He also presented bilateral papilledema, hyporeflexia of the pupils, dysarthria, generalized pallor, bilateral presbyacusis, and multiple (numerous) fibroids (Figure 1).
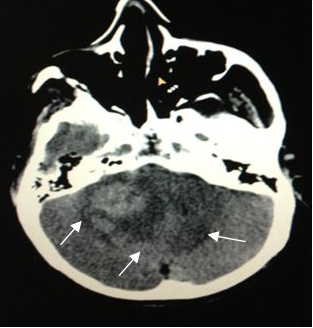
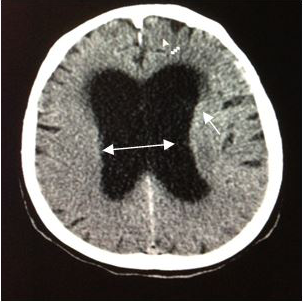
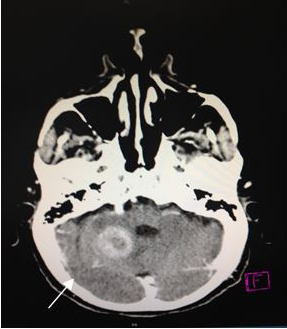
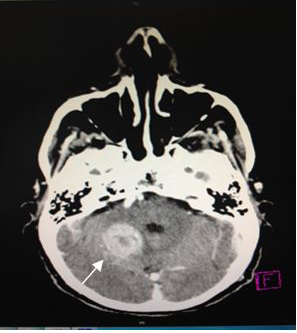
On admission, a simple skull computed tomography was performed, showing a hyper dense heterogeneous tumor in the posterior fossa, with peritumoral edema. In addition, a peripheral hypo dense area in the right cerebellar hemisphere was observed, which one that was making compression of the perimesencephaliccisterns. Likewise, an increase in the size of the ventricular system was observed, accompanied by subependymal edema (Figures 2) (Figure 3), leading to the radiological diagnosis of an acute non-communicating hydrocephalus. Computed tomography with contrsast of the skull was performed, showing heterogeneous lesion located in the cerebellar hemisphere (Figures 4) (Figure 5).
|
Figure 3 CT scan of theskull, axial section (5mm).Increase in the size of the ventricular system, with subependymal edema → Radiologic diagnosis of an acute non-communicating hydrocephalus. |
|
Figure 4 CT scan of the skull, axial section (5 mm). A heterogeneous image, with hypodense center and moderate reinforcement, in the right cerebellar hemisphere. |
|
Figure 5 CT scan of the skull, axial section (5 mm).A heterogeneous image that reinforcement after the administration of contrast medium with necrotic center, it is pointed by the arrow. |
A ventriculo-peritoneal shunt valve was placed without complications, resolving acute hydrocephalus and allowing the patient’s placement to the intensive care unit for postoperative monitoring.
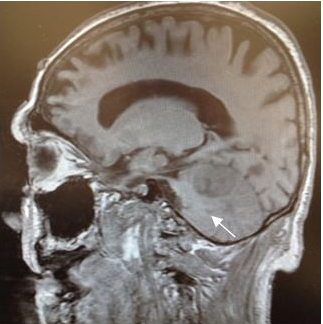
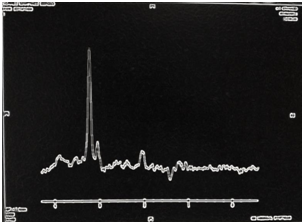
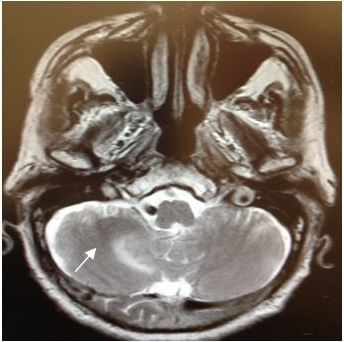
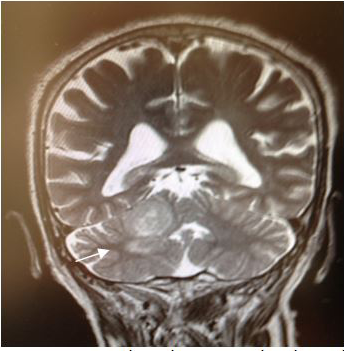
Magnetic Resonance (1.5T) of the skull was performed with a contrast medium. Axial, coronal and sagittal slices, in sequence T1 and T2 were acquired (Figures 6–8). Axial T1 images with Gadolinium as contrast medium were also carried out (Figure 9). In addition it was made a spectroscopy with univoxel technique with TE of 144 msto see the peeks of N-acetylaspartate, creative, chorine and lactate (Figure 10).
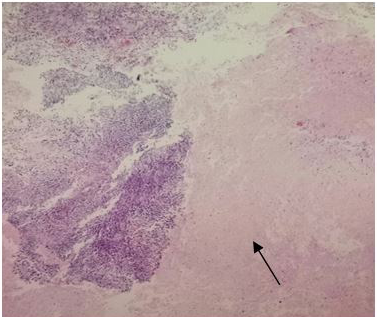
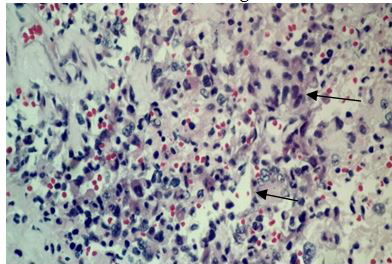
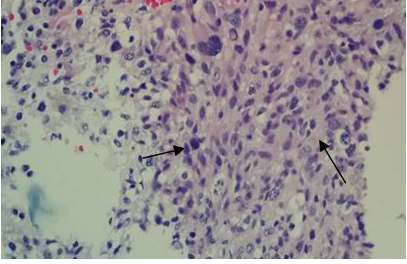
The surgical procedure for tumor removal (partial resection of the tumor) and later on for histopathological examination, consisted of a subtemporaltranstentorial approach with conventional microsurgical technique, without complications. However, 6days after the surgical procedure, the patient presented generalized deterioration, becoming complicated with nosocomial pneumonia and despite treatment based on an antibiotic therapy, oxygen therapy with support of mechanical ventilation, and measures against cerebellar edema, the patient finally presented cardio respiratory arrestanddied without having been given an adjuvant treatment. The histopathological examination of the surgical specimens diagnosed a Glioblastoma (Figures 11A–C).
|
Figure 6 Magnetic Resonance imagingof the skull, in the axial T2 sequence, where a heterogeneous lesionwith perilesional edema, located in the right cerebellar hemisphere, is observed. |
|
Figure 8 Coronal T2 image T2 showing a heterogeneous lesion with perilesional edema, located in the right cerebellar hemisphere, adjacent to the tentorium cerebelli. |
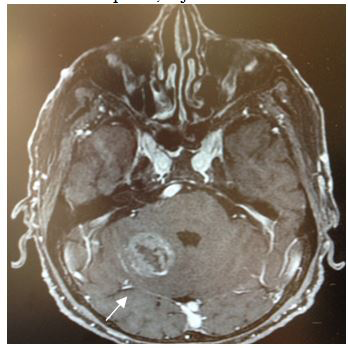
|
Figure 9 Axial T1 image after contrast enhancement. Shows an heterogeneous tumor reinforcement, in the right cerebellar hemisphere with hypointense and necrotic center. |
Conclusion
Glioblastomas of the posterior fossa remain very rare tumors.1,9 Our team takes the opportunity to present thiscase publication, since this is the first report (in our database) of a primary Glioblastoma (WHO Grade IV) in an adult with a cerebellar localization. In our case, the time between diagnosis and the onset of complications was very short, thus could not be put in the average survival time of 6-8 months as mentioned in some publications.5 The symptoms presented by the patient were more frequent (nausea, headache, vomiting, ataxia, confessional state) as mentioned in the literature.10,11
Differential diagnoses in posterior fossa lesions should consider metastatic lesions, cerebellar infarction, cystic astrocytomas, abscess, hemangioblastoma, and entities such as encephalitis, tuberculoma, or multiple sclerosis.12,13 Patients with a history of NF1 have a 5 times higher risk of developing glioblastoma than the general population.8
Spectroscopy of astrocytomas shows a reduction in NAA due to loss of neuronal elements, and elevation of the peak of Choline that may reflect the increase in membrane synthesis, cell membrane proliferation. The presence of the lactate peak correlates with the high degree of malignancy observed in Glioblastomas.14,15
Acknowledgements
None.
Conflicts of interest
The author declares no conflict of interest.
References
- Mustafa Kemal Demir, Tayfun Hakan, Akinci O, et al. Primary cerebellar glioblastoma multiforme: Turkish Society of Radiology 2005. Diagn IntervRadiol. 2005;11:83–86.
- Russell DS, Rubinstein LJ. Pathology of tumors of the nervous system. 5th ed. Baltimore: Williams & Wilkins; 1989. p. 219–247.
- Hur H, Jung S, Jung TY, et al. Cerebellar glioblastoma multiforme in an adult. J Korean Neurosurg Soc. 2008;43(4):194–197
- Ferlay J, Parkin DM, Steliarova –Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46(4):765–781.
- Adams H, Chaichana KL, Avendanõ J, et al. Adult cerebellar glioblastoma: understanding survival and prognostic factors using a population–based database from 1973 to 2009. World neuro surgery. 2013;80(6):e237–e243
- Vanderberg S. Current diagnostic concepts of astrocytic tumors. J Neuro pathol Exp Neurol. 1992;51(6):644–657.
- Kuroiwa T, Numaguchi Y, Rothman MI, et al. Posterior fossa glioblastoma multiform: MR findings. AJNR Am J Neuro radiol. 1995;16(3):583–589.
- Jaishri O. Blakeley, Scott R, et al. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro–Oncology. 2016;18(5):624–638.
- Katz DS, Poe LB, Winfield JA, et al. A rare case of cerebellar glioblastoma multiforme in childhood: MR imaging. Clin Imaging. 1995;19(3):162–164.
- Rosenfeld J, Rossi ML, Briggs M. Glioblastoma multiforme of the cerebellum in an elderly: a case report. Tumori. 1989;75(6):626–629.
- Occhiog Rosso M, Spada A, Merlicco G, et al. Malignant cerebellar astrocytoma: report of five cases. J Neuro Surg Sci. 1985;29(1):43–50.
- João Paulo Mattos, Horacio Armando Marenco, et al. Cerebellar glioblastoma multiforme in an adult. Arq Neuropsiquiatr. 2006;64(1):132–135.
- Fulham MJ, Bizzi A, Dietz MS, et al. Mapping of brain tumor metabolites imaging clinical relevance. Radiology. 1992;176:509–515.
- Ping H. Lai, Jih T Ho, et al. Brain abscess and necrotic brain tumor: discrimination with proton MR spectroscopy and diffusion–weighted imaging. American Journal of Neuroradiology. 2002;23(8):1369–1377.
- Panigrahi S, Mishra SS, Das S. Primary cerebellopontine angle glioblastoma in an adult. Asian Journal of Neurosurgery. 2017;12(1):62–64.