Volume : 1 | Issue : 2
Research
Prevotella denticola protease interferes with Porphyromonas gingivalis Lipopolysaccharide mediated secretion of anti-inflammatory components by the cultured monocytic cells
Jegdish P Babu,1 Jason Primm2
1Departments of Bioscience Research, College of Dentistry, University of Tennessee Health Science center, USA
2Department of Periodontology, College of Dentistry, University of Tennessee Health Science center, USA
Received: July 09, 2018 | Published: July 25, 2018
Abstract
Aim of investigation: Principal aim of this study is to investigate the role of periodontal pathogen protease on the secretion of anti-inflammatory components by cultured monocytic cells in response to Porphyromonas gingivalis lipolysaccharide (Pg-LPS) stimulus.
Methods: Primed, cultured THP-1 leukemia cells were incubated briefly with isolated protease of Prevotella denticola (Pd-protease) and then challenged with Pg-LPS. The cel culture supernatants containing Secretory leukocyte protease inhibitor (SLPI) and Interleukin-10 cytokine were quantitated by ELISA kits specific for these two components.
Results: Pg-LPS stimulated the secretion of SLPI and IL-10 by the THP-1 cells several folds compared to the control. Pre-incubation of THP-1 cells with Pd-protease a significant (p=0.0027) reduction in the secretion of both SLPI and IL-10 was observed. Approximately 57% to 84% reduction in secretion was observed. When the cells were incubated with heat inactivated Pd-protease there was no inhibition.
Conclusion: The results of the study suggest that the bacterial components, such as proteases released by the biofilm microorganisms of the oral cavity exerts direct effect on the interaction of LPS of periodontal pathogens with host monocytic cells.
Keywords: cytokines, lipopolysaccharide, monocytic cells, periodontal pathogens, protease, SLPI.
Abbreviations
LPS, Lipopolysaccharide; Pg, Porphyromonas gingivalis; Pd, Prevotella denticola; ELISA, Enzyme linked immunosorbent assay; PMA, phorbol 12-myristate 13-acetate; SLPI, Secretory leukocyte peptidase inhibitor Interleukin-10 (IL-10); pg, Picograms
Introduction
Periodontal disease is a major public health problem, it is initiated by the formation of mixed bio film on teeth and gum tissues. The oral cavity contains over 700 species of microorganisms, however, only a few of these have been consistently been associated with periodontal disease when their numbers increase several folds.1 Lipopolysaccharide (LPS) of these periodontal pathogens been known to play a key role in the disease as were shown to induce an inflammatory response by upregulating secretion of various cytokines, and some of them promotes the progression of the disease by their interaction with immune cells.2 Macrophage and other host cell products such as cytokines are released as a consequence of high levels LPS challenge were found to promote systemic inflammatory response.3 In addition to LPS, other virulence factors such as proteases secreted by these pathogens were identified as contributing factors in disease progression. Among the oral pathogens, P. gingivalis interaction with host cells have been widely investigated, the bacterial LPS and other cellular proteases also shown to induce various cytokines, some of them are anti-inflammatory in nature but most of them are pro-inflammatory.2
Secretory leukocyte protease inhibitor (SLPI), a cysteine-rich cationic protein secreted by various cells. The main function of SLPI is to protect local tissue against the detrimental consequences of inflammation and by inhibiting the proteases secreted by oral pathogens4 and was found in saliva and various other body fluids.5 LPS of P. gingivalis was shown to induce higher levels of SLPI and IL-10 an anti-inflammatory cytokine by the phagocytic cells in addition to various other cytokines.6 The vital role of LPS in periodontal disease is well understood but the role of other microbes especially other periodontal pathogens and their products that are present in the biofilm on the LPS-mediated interactions are not well understood. The purpose of this investigation is to investigate the influence of an isolated crude protease from Prevotella denticola, a periodontal pathogen on P. gingivalis LPS interaction with cultured monocytic cells on the secretion of anti-inflammatory components, SLPI and IL-10.
Materials and methods
Cultured monocytic cells
THP-1 (ATCC TIB202), originally isolated from a child with acute leukemia, are mature cells in the monocyte/macrophage lineage with a normal diploid karyotype, and they produce TNF-α and other cytokines in response to purified endotoxin.7 These non-adherent cells were maintained in continuous culture with RPMI 1640 (GIBCO/BRL, Grand Island, N.Y.), 10% fetal bovine serum (GIBCO/BRL), and 0.05mM 2-mercaptoethanol (GIBCO/BRL) in an atmosphere of 5% CO2 at 37°C for 24hours. THP-1 cells were collected by centrifugation, washed three times with culture medium without fetal bovine serum and re suspended to a concentration of (1x106perml). The cells were plated in a 48-well culture plate (Corning; Fisher Scientific, Norcross, GA, USA) and then treated with phorbol 12-myristate 13-acetate (10-7M; Calbiochem Co., La Jolla, Calif.) to induce maturation of the monocytes. The differentiated cells adhered to the plastic and microscopic observation showed them to be like macrophages. Cell viability of the adherent cells was determined to be 95% by the trypan blue dye exclusion assay.
LPS Preparation
The bacteria, P. gingivalis (25260) was obtained from ATCC, Rockville, MD. The bacteria were grown in one-liter quantities in thioglycollate broth (Difco) for 72hours in an anaerobic jar. The bacteria were harvested by centrifugation and the cell pellets were washed with physiological saline, then with de-ionized water and centrifuged. The bacterial cell pellets were lyophilized and the LPS was prepared by the hot phenol-water extraction method as described by Westphal and Jann8. Batches of 0.25g of the lyophilized bacteria were suspended in 200ml of 90% phenol and water and incubated at 67°C for 15minutes and then stirred for another 15minutes on a stirrer at room temperature. The mixture was chilled on ice to 10°C to separate the phases. The resultant LPS-containing aqueous phase was then collected from the lower phenol phase. The non-aqueous phenol phase was subjected to a second extraction by adding an additional 200ml of water. The pooled aqueous phase was dialyzed against distilled water at 4°C for 48hours. The aqueous phase was collected and frozen using liquid nitrogen and then lyophilized. The purity and lack of protein contamination of the isolated LPS was confirmed by gel electrophoresis (10% polyacrylamide gel containing 0.1% sodium dodecyl sulfate.9 LPS samples were sonicated gently prior to electrophoresis, and then the gel was stained with silver nitrate stain10 and the endotoxin activity of the LPS samples was confirmed with the Limulus amoebocyte assay.11
Quantitation of secreted SLPI and IL-10
SLPI and IL-10 quantikine ELISA kits were obtained from R & D systems (Minneapolis, MN). The quantifications of SLPI and IL-10 was performed according to the method described by the manufacturer.
Preparation of P. denticola protease
Prevotella denticola (ATCC 33185) was grown in 100ml thioglycollate broth in anaerobic jar for 72hours. Bacterial cells were pelleted by centrifugation at 8,000 xg for 20min at 4°C. The bacterial pellet was suspended (15mg/ml) in 50mM Tris-HCl-0.2 M NaCl-5mM CaCl2 (Tris buffer) pH 7.5. The pellet was subjected to sonication four times for 10s each, and then the sonicate was pooled and centrifuged at 10,000xg for 20min. The collected supernatant was filtered through a 0.22μm Millipore filter (Med Lab supply, Pompano Beach, FL, USA) and then concentrated through a 100-kDa filter using a tangential flow filtration Minimate TFF System (Pall Corporation, Port Washington, NY, USA) according to the manufacturer’s instruction. The Pd-protease preparation was freeze-dried and suspended to the desired concentration for use in the experiments. The biological activity of Pd-protease was confirmed using Pierce Protease assay kit according to the recommended protocol by the manufacturer (Thermo Scientific (Rockford, IL, USA) which utilizes the succinylated casein as the substrate.
Statistical analysis
All assays were performed in triplicates and each experiment was repeated three times. The significance (P<0.05) of the data was analyzed by Statview software. ANOVA with post-hoc analysis was performed for comparison of the cells treated with different components and the data presented as mean±standard error.
Results
Demonstration of P. denticola protease activity
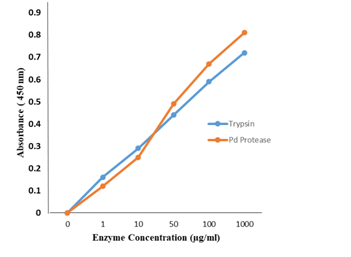
Using the Pierce Protease assay kit, we tested the protease activity using succinylated casein substrate. Figure 1 shows the protease activity of the isolated protease found to be comparable to the standard enzymatic activity of the assay kit.
Effect of P. gingivalis LPS on the secretion of SLPI and IL-10 by THP-1 cells.
Triplicate samples of PMA-primed THP-1 cells (106) plated in a 48-well culture dish were incubated with varying amounts of P.gingivalis LPS (1,5 and 10µg/ml) for 12hours. Culture supernatant from each well was collected and assayed for secreted SLPI and IL-10 by ELISA using the quantikine ELISA kits from R & D labs according to the manufacturer’s recommended method. The results of Table 1 show that the LPS stimulated the monocytic cells to secrete significantly higher amounts (P=0.0261 and P=0.018) of SLPI and IL-10, respectively compared to the control. The increase was found to be dose-dependent upon the amount of LPS used in the assay. Cells treated with 1µg/ml of LPS secreted 94.6±22.4pg/ml of SLPI and 67.9±1pg/ml of IL-10. When treated with 5µg/ml of LPS a 3-fold increase was seen in the SLPI secretion. Similarly, 2.5-fold increase in IL-10 secretion. Slightly higher amounts of SLPI and IL-10 was secreted when cells were incubated with 10 µg/ml LPS, but the increase was not significant (P=0.072; Table 1).
Cells incubated with LPS (µg/ml) |
SLPI (pg/ml)±SE |
IL-10 (pg/ml)±SE |
|
|---|---|---|---|
0.0 (Control) |
36.4±12.6 |
10.4±3.3 |
|
1.0 |
94.6±22.4 |
67.9±13 |
|
5.0 |
306.3±54.3ƪ |
166.7±24.9ƪ |
|
10.0 |
|
322.5±41.6ƪ |
188.3±21.8ƪ |
Table 1 Effect of Pg-LPS Stimulation of cultured monocytic cells on the secretion of SLPI and IL-10
The data are presented as averages from three separate experiments with triplicate samples. Each error bar indicates the standard error of the mean (SE).
All three concentrations of LPS enhanced the secretion of both SLPI and IL-10 significantly (P<0.05).
ƪNo significant difference between the cells treated with 5 or 10µg/ml of LPS.
THP-1 monocytic cells when incubated with heat-inactivated (80°C for 10min) P. denticola protease (100µg/ml) had no effect on LPS-stimulated secretion of SLPI and IL-10. In fact, there was a slight decrease though not significant, in the secretion of SLPI and IL-10 (data not shown).
Effect of P. denticola protease on LPS interaction with THP-1 cells:
In order to investigate if the protease component of other periodontal pathogen effect on LPS stimulation of cultured monocytic cells, primed THP-1 cells (1x106cells) plated in the culture plate were incubated with 50µg/ml of the isolated Pd-protease (50µg/ml) for 10min. Cells were rinsed with Tris buffer twice. The cells were then incubated with Pg-LPS as described above. Culture supernatants were collected and measured the amounts of secreted SLPI and IL-10 as described previously. We also tested for the cell viability following the incubation with Pd-protease by trypan blue exclusion assay and found that the 95% of the cell were viable.
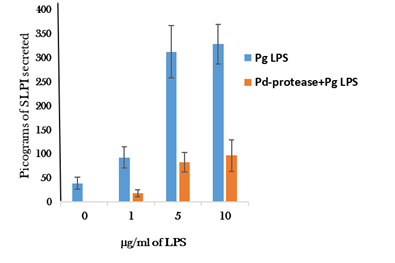
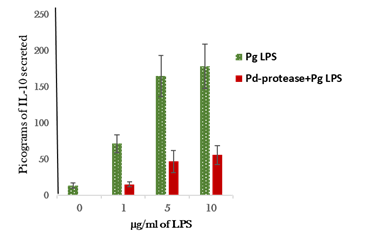
Pd- protease incubation of the cells had a significant inhibitory effect on Pg-LPS stimulation of the cultured monocytic cells to secrete SLPI and IL-10 (Figures 1) (Figure 2). Heat inactivation of the protease preparation (80°C for 15min) abolished the inhibitory effect (data not shown).
Cultured monocytic cells incubated with Pd-protease significantly inhibited LPS-induced SLPI at all the three concentrations tested (Figure 1). Approximately 79% to 71.5% suppression in SLPI secretion was observed when stimulated with 1.0µg/ml and 10µg/ml LPS (P=0.023;0.017), respectively. There was no significant difference in SLPI secretion (P=0.31) between cells incubated with 5µg/ml or10µg/ml of Pg-LPS (Figure 3).
Pd-protease treatment of the monocytic cells also suppressed the secretion of anti-inflammatory cytokine, IL-10 when stimulated with Pg-LPS (Figure 2). Approximately 81% decrease in IL-10 secretion from the cells stimulated with 1µg/ml of LPS when compared to the IL-10 secretion in the absence of protease treatment. In a similar fashion, 70-72% decrease in IL-10 secretion by the cells incubated with 5-10µg/ml of LPS (Figure 2). Collectively, these results suggest that the protease of periodontal pathogen, P. denticola has a potent inhibitory effect on the secretion of anti-inflammatory cytokines by Pg-LPS-stimulated cultured monocytic cells, this effect may play a role in progression of inflammatory reaction associated with periodontal disease by suppressing the anti-inflammatory components such as SLPI and IL-10 cytokine.
Discussion
Bacterial LPS especially that of periodontal pathogens is the most potent stimulator of immune cells. Two members of the Toll-like receptor (TLR) family, TLR2 and TLR4, have been identified as possible signaling receptors for bacterial cell wall component such as LPS.12 Products released after LPS interaction with immune cells of the body are both anti- and pro-inflammatory in nature depending on their concentration, at high levels it was shown to induce various pro-inflammatory cytokines.13 Among the important anti-inflammatory protein released by monocytes in response to LPS is SLPI, an 11.7-kDa cationic protein and a member of the innate immunity-associated proteins present in saliva and various other body fluids.5,14 SLPI shields the tissues against inflammatory products by down-regulating the macrophage responses against LPS. It was reported that SLPI suppresses the monocytic cells ability to release pro-inflammatory cytokines.15 The IL-10 is a key cytokine capable of inhibiting pro-inflammatory responses secreted under different conditions of immune activation by a variety of immune cells in response to LPS.16
The results of our study demonstrated that both SLPI and IL-10 secretions by cultured monocytic cells are stimulated in response to P. gingivalis LPS consistent with other studies.17 Most of the studies were conducted with bacterial LPS alone which does not include the role of other bacterial products present in a biofilm. Identifying the contribution of other bacterial components or their products are crucial in understanding the periodontal disease, since the disease is not caused by a single pathogen. The current study was designed to investigate if the other bacterial component (s) exerts any effect on LPS interaction with monocytes. The results of our study clearly demonstrated that the crude protease isolated from the outer membrane of periodontal pathogen, P. denticola suppressed the secretion of anti-inflammatory SLPI and IL-10 by the cultured monocytic cells in response to periodontal pathogen, P. gingivalis LPS. Reduction in SLPI and IL-10 secretion accelerates the inflammatory responses associated with periodontal disease. The exact mechanism by which the P. denticola protease suppresses these anti-inflammatory components in response to low levels of periodontal pathogen LPS is not known. It was shown that the proteases (gingipains) of P. gingivalis denatured the LPS-binding protein on the phagocytic cells.18 The bacterial proteases were also shown to cleave CD14 and LPS binding protein and as a result of this the LPS interaction with monocytes is suppressed.19 The findings of the present study, suppression of SLPI and IL-10 levels in response to low levels of LPS by P. denticola protease may be due to its effect on the LPS binding site or they may play a direct role in de-naturing the LPS itself, the scenario is less likely given the chemical nature of LPS. These possibilities need to be determined to understand the underlying mechanism in LPS-induced inflammatory conditions and products of other microorganisms of the dental plaque involved in the progression of periodontal disease.
Acknowledgements
None.
Conflict of interest
The author declares no conflict of interest.
Reference
- Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontol. 2006;42:180–218.
- Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis epithelial cell interactions in periodontitis. J Dent Res. 2006;85(5):392–403.
- Marino MW, DunnA, Grail D, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci. 1997;94(15):8093–8098.
- Doumas S, Kolokotronis A, Stefanopoulos P. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun. 2005;73(3):1271–1274.
- Pillay K, Coutsoudis A, Agadzi-Naqvi AK. Secretory leukocyte protease inhibitor in vaginal fluids and perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;183(4):653–656.
- Daigneault M, Preston JA, Marriott HM, et al. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 Cells and monocyte-derived macrophages. PLoS one. 2010;5(1):e8668.
- Bosshart, Herbert, Heinzelmann M. THP-1 cells as a model for human monocytes. Ann Transl Med. 2016;4(21): 438.
- Westphal O, Jann K. Bacterial lipopolysaccharides; extraction with phenol- water and further applications of the procedure, in: R.L. Whistler editor. Methods in carbohydrate chemistry. New York: Academic Press Inc: 165;5:83–91.
- Laemmli UK. Cleavage of Structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685.
- Tsai CM, Frasch CE. A sensitive silver stain of detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119(1):115–119.
- Obayashi T, Tamura H, Tanaka S, et al. A new chromogenic endotoxin-specific assay using recombined limulus coagulation enzymes and its clinical applications. Clin Chim Acta. 1985;149(1):55–65.
- Yoshimura A, Kaneko T, Kato Y, et al. Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human toll-like receptor. Infect Immun. 2002;70(1):218–225.
- Jin F, Nathan CF, Radzioch D, et al. Lipopolysaccharide-related stimuli induce expression of the secretory leukocyte protease inhibitor, a macrophage-derived lipopolysaccharide inhibitor. Infect Immun. 1998;66(6):2447–2452.
- Pillay K, Coutsoudis A, Agadzi-Naqvi AK. Secretory leukocyte protease inhibitor in vaginal fluids and perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;183(4):653–656.
- Hiemstra PS. Novel roles of protease inhibitors in infection and inflammation. Bio chem Soc Trans. 2000;30(2):116–120.
- Murthy PK, Vida A Dennis, Barbara L Lasater, et al. Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with borrelia burgdorferi lipoproteins. Infect Immun. 2000;68:6697–6703.
- Pulendran B, Kumar P, Cutler CW, et al. lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167(9):5067–5076.
- Into T, Inomata M, Kanno Y, et al. Arginine-specific gingipains from porphyromonas gingivalis deprive protective functions of secretory leucocyte protease inhibitor in periodontal tissue. Clin Exp Immunol. 2006;145(3):545–555.
- Cecil JD, O’Brien-Simpson1 NM, Lenzo JC, et al. differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens. PLoS one. 2016;11(4):e0151967.