Volume : 2 | Issue : 2
Research
Lactobacillus rhamnosus versus Staphylococcus aureus: influence on growth and expression of virulence factors
Fornitano ALP,Amêndola I, Santos SSF, Silva CRG, Leão MVP
Instituto Básico de Biociências, Faculdade de Medicina, Universidade de Taubaté, Brazil
Received: February 05, 2019 | Published: March 01, 2019
Abstract
Staphylococcus aureus is one of the main etiological agents of community and hospital infections. Due to the virulence factors of this species and the multiresistance of some strains, diseases caused by S. aureus are difficult to treat. Numerous therapeutic alternatives have been studied and, among them, probiotics stand out. In this context, the objective of the present work was to analyze the action of Lactobacillus rhamnosus on growth and virulence factors of S. aureus. Inhibitory activity was evaluated by the two-layer plate method. L. rhamnosus was seeded in MRS agar and incubated at 37°C and CO2 for 24h. Subsequently, BHI agar was added to the medium, and S. aureus was seeded, and the plates were incubated for 24, 48 and 72h. S. aureus strains isolated from co-culture with L. rhamnosus were investigated for coagulase production in horse plasma, by observation of clot formation, haemolysin production, by visualization of hemolysis in blood agar, and for biofilm formation in microliter plates. The results were compared to the S. aureus strains cultured in the absence of L. rhamnosus. The results showed that there was an inhibitory activity of L. rhamnosus on S. aureus, as well as a significant reduction in the production of coagulase, in all analyzed periods. It was also observed that after 72h of co-cultivation with L. rhamnosus, S. aureus significantly reduced the biofilm formation capacity. Thus, the results of the present study demonstrated that L. rhamnosus is able to inhibit S. aureus growth and to interfere with coagulase production and biofilm formation, suggesting a possible beneficial use of these probiotic bacteria in the prevention and / or treatment of staphylococci diseases.
Keywords: virulence factors, probiotics, Staphylococcus aureus
Introduction
Staphylococcus aureus is one of the most common aetiologic agents of infections acquired in the community and in hospital environments.1,2 This bacteria is part of the transient microbiota of the skin and mucosa, mainly in hot and humid regions, such as the groin, axilla and nasal cavity.3 In addition, S. aureus is considered an important opportunistic pathogen causing superficial skin infections, such as impetigo, until deep infections, as bacterial endocarditis, osteomyelitis and toxic shock.4
The virulence factors of S. aureus are closely related to its pathogenicity. The presence of membrane proteins, enzyme production (coagulase and hemolysin), escape from the cells of the immune system, biofilm production and antimicrobial resistance are some of the factors of virulence of S. aureus, favoring its colonization, infection and dissemination in the host tissues.5,6
aureus produces two types of coagulase: the classical coagulase and the binding protein Von Willibrand factor, which are able to convert fibrinogen into fibrin. S. aureus can also interact directly with fibrinogen to form large groups of cells, mediated by cell surface proteins. These virulence mechanisms provide better adherence to host cells, and also impair the phagocytosis by cells of the immune system.7,8
One of the main mediators of cell death induced by S. aureus is alfa haemolysin, a toxin that open pores in the target cell membrane.9 Haemolysin is also related to invasion of host cell and adhesion on surfaces of catheters and prostheses.7
aureus is also able to form biofilms, which is an association of microbial cells surrounded by an extracellular polysaccharide matrix10. The presence of microorganisms in biofilm is common in nature and contribute to its resistance to the action of immune system cells and antimicrobial substances.11-13
Studies aiming the discovery of alternative therapies for the treatment of infections caused by multidrug-resistant strains have become increasingly frequente. For this purpose, probiotics have been studied.14-16
According to the World Health Organization (WHO),17 probiotics are living microorganisms that promote health benefits to the host when used in adequate amounts. Lactobacillus and Bifidobacterium strains are among the most importante bacteria.18
Probiotic strains can develop mutualism with pathogenic strains, preventing the proliferation of pathogens, as well as the development of diseases.19,20 In vitro studies show that Lactobacillus strains are able to increase phagocytic activity of human macrophages against extracellular pathogens such as S.21,22 In addition, Lactobacillus can decrease cellular proliferation of S. aureus by reducing the pH, due to formation of products of fermentative activity, producing of antimicrobial substances (bacteriocins) and competing for nutrients and adhesion sites.23,24
Since the knowledge about the relationship between Lactobacillus and S. aureus can contribute to the elaboration of strategies for the prevention and/or treatment of staphylococci diseases, the present work aimed to verify the action of living cells of Lactobacillus rhamnosus on the growth and expression of virulence factors by S. aureus.
Material and methods
Microorganisms
Staphylococcus aureus(ATCC 25923) was plated on Mannitol Salt Agar (Biolog,Hayward, USA) for 24h at 37°C. Aliquots from the colony formin units were added in sterile saline solution (NaCl 0.9%) until obtaining suspensions of 106 cels.ml-1, standardized in spectrophotometer at 490 nanometers (nm).
Lactobacillus rhamnosus(ATCC 1465) was plated on Man-Rogosa-Shape agar(MRS, Himedia, Mumbai, India) for 48h at 37°C and 5% CO2 and suspensions of 107 cels.ml-1 were obtained in saline solution, standardized at 540 nm.
Inhibitory activity of L. rhamnosus on S. aureus
Twenty microliters of standardized suspension of L. rhamnosus or 20uL of sterile physiological solution (control) were pipetted in single points on the surface of a Petri dishes containing 15 ml of MRS agar. The plates were incubated at 37°C at 5% CO2 for 24h. After this period, 15 ml of BHI agar were added over the MRS agar with growth of L. rhamnosus. After solidification, 0,1 ml of the standard suspension of S. aureus weresown with the aid of Drigalski handle, and the plates were incubated at 37°C for 24h, 48 h or 72h in aerobic environment. After incubation, the presence of inhibition halos was checked. Analysis of inhibition was conducted according to the methodology proposed by Tagg et al..25
After halo measurement, aliquots of S. aureus from colonies, located within a radius of 5 mm close to the halo of inhibition, were collected for investigation of virulence factors.
The experiments were conducted in four independent trials (n=4) and in triplicate, totalling n=12.
Analysis of coagulase production
The aliquots of microorganisms were seeded in Mannitol Agar and incubated at 37ºC for 24h, for isolation of S. aureus. After that, suspensions were standardized in spectrophotometer in accordance with the methodology described previously.
Aliquots of 0,3ml of standard suspensions were transferred to sterile tubes containing 0,3 ml of horse plasma. After incubation at 36±°C for 6h, the formation of clots was verified, considering the following criteria: no clot formation (negative reaction); small and disorganized clot (+); small and organized clot (++); big and organized clot (+++); coagulation of the entire contents of the tube (++++).26
Analysis of haemolysin production
For the test of the hemolytic activity, it was used blood agar Base added with 5% of horse blood. Aliquots of 3ul of standard suspensions of S. aureus were sown in this medium and after incubation for 24h at 37°C the occurrence of translucent halo around the colonies was checked, indicating positive hemolytic activity.
Enzyme activity (Pz) was evaluated by the ratio of the diameter of the colony and the diameter of the colony plus the zone of hemolysis. The smaller the value of Pz, the greater the enzyme activity. The enzyme activity was classified in: negative (Pz=1), positive (≥0.64 Pz< 1) and strongly positive (Pz<0.64).
Formation of biofilm
In microplates of 96 wells, 100µl of the standard suspensions of S. aureus and 100µl of BHI broth (Brain Infusion Hearth) doubly concentrated were added. The plates were incubated at 37°C with agitation for 180 min initial adhesion of cells. After this period, the contents of the wells were removed and the wells were washed three times with 200µl of sterile saline solution for removal of not attached cells. After, 200µl BHI broth were added to the wells and the plates were incubated at 37°C for 24h with agitation, for the formation of biofilms. After incubation, the contents of the wells were removed and washed three times with 200µl sterile physiological solution. The optical densities of the biofilms formed on the bottom of the wells were verified in microplate reader at 530 nm.
Subsequently, with the aid of sterilized sticks, the bottom of each well was scraped, with movements in various directions, for 30 seconds. After that, 100µl of the contents of each well were transferred to sterile microtube containing 900µl of sterile saline solution, and homogenized for 30 seconds. From this suspensions, serial dilutions were performed and 20µl of each one were seeded in Mannitol agar plates and incubated at 37°C for 24h. After the incubation period, the Colony Forming Units per milliliter (CFU/ml) were determined.
Statistical analysis
The results were analyzed for normality. The results of inhibitory activity were analysed using ANOVA and Tukey test. For the enzyme coagulase, t test was used. The other results were analysed by Kruskal Wallis test and Dunn's test. All results were analysed using the program GraphPad Prism 5.0 (GraphPad Software Inc.), considering a significance level of 5%.
Results
Inhibitory activity of rhamnosus on S. aureus
After 24, 48 and 72h of incubation, it was observed the presence of inhibition halos of S. aureus growth around the colony of L. rhamnosus.
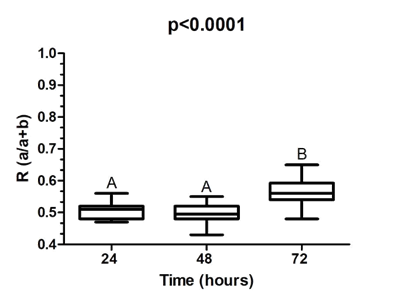
Figure 1 represents the ratio between the diameter of the colony of L. rhamnosus and the diameter of the colony of L. rhamnosus plus the halo of inhibition (a + b) of S. aureus after co-culture of these species. The lower the value, the higher the inhibitory activity of L. rhamnosus. In this way, after 24h and 48h, statistically grater inhibitory activity of L. rhamnosus on S. aureus (p<0.0001) was observed.
Coagulase
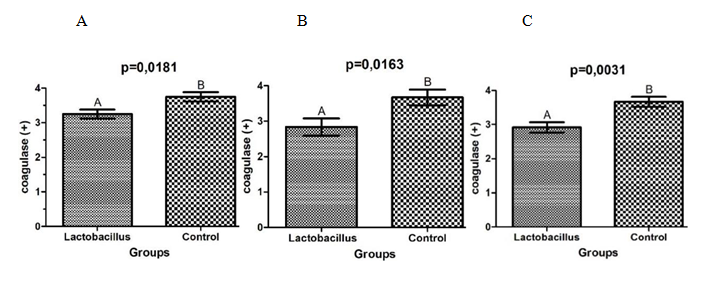
There was a significant reduction in the production of coagulase by S. aureus isolated from co-cultures with L. rhamnosus, when compared to the control group (Figure 2). Comparing the percentage of reduction, with 48 h of association the lower production of the enzyme was observed (22.73%), followed by 20.45% in 72h and 13.33% in 24h.
Haemolysin
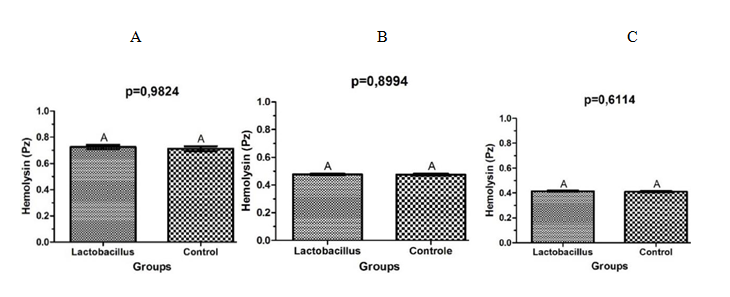
After analysis of the halos of hemolysis, it was observed that there was no statistically significant difference in the activity of hemolysin of strains of S. aureus cultivated in presence of L. rhamnosus when compared to the control group, independent of the time of interaction. Figure 3 shows the average of the values of Pz found in 24, 48 and 72h.
Formation of biofilm
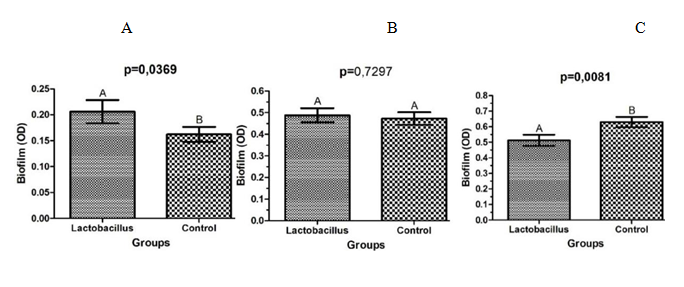
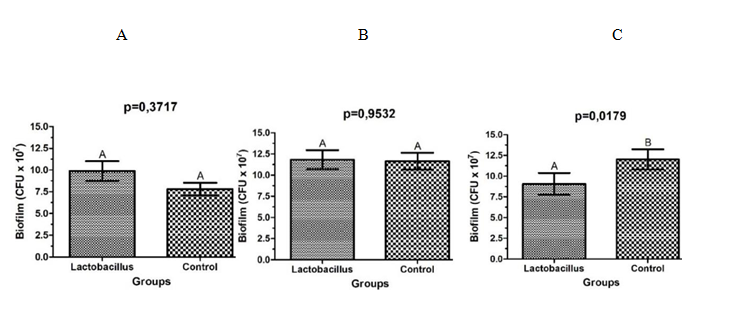
After the analysis of the optical densities of the biofilms of S. aureus in microplates of 96 wells, it was observed that the strains which were in the presence of L. rhamnosus for 24h, showed an increase in biofilm formation capacity (p=0.0369), when compared to the control group (Figure 4A). After 48h of co-culture, there was no statistical difference between the groups (p=0.7297) (Figure 4B). Instead that, with 72h of contact with L. rhamnosus, S. aureus reduced significantly its capacity of biofilm formation (p=0.0081) (Figure 4C).
After CFU/mL counting from S. aureus biofims, it was observed a reduction in the number of cells of S. aureus wich were in contact with L. rhamnosus for 72h (p=0.0179), when compared to the control group (Figure 5C). Statistically significant differences were not observed in CFU/ml counts of biofilms of S. aureus isolated from cultures in the presence of L. rhamnosus for periods of 24 (p=0.3717) and 48 h (p=0.9532) (Figure 5 AB).
Discussion
The results obtained in this study suggest a antagonism between L. rhamnosus and S. aureus, since inhibition was observed in all tests, with greater inhibitory activity during the 24 and 48 h period of co-existence. As previously reported by other authors, Lactobacillus reduces the growth of S. aureus, through pH reduction resulted from fermentative activity, production of antimicrobial substances (bacteriocins) and competition.23,24 The smaller inhibition halo observed after 72h of co-culture probably resulted from the exhaustion of the effects of lactobacilli products which were capable to interfere with S. aureus growth.
Bertuccini et al. (2017)27 found similar results when they studied the antimicrobial activity of L. rhamnosus and L. acidophilus on S.aureus, Gardenerella vaginalis, Atopobium vaginae and Escherichia coli, also using in vitro assays of co-culture. Glück and Gebbers28 had already verified in vivo this antagonism. The authors observed that the ingestion of fermented milk containing L. rhamnosus, Bifidobacterium sp., L. acidophilus and Streptococcus thermophilus reduced significantly the occurrence of Gram-positive bacteria, including S. aureus, in nasal microbiota.
The antimicrobial activity of lactobacilli against S. aureus presented in biofilms was also demonstrated,29,30 confirming that this effect is independente of staphilococci condition and of the methodology used.
The present study demonstrated that L. rhamnosus was also able to interfere with the production of coagulase by S. aureus. This enzyme was one of the first factors of virulence of S. aureus described and is considered one of the most importante one. The coagulase linked to bacteria cell protects from the host immune system and the secreted coagulase is responsible for the formation of a fibrin clot that also protects from phagocytosis.31
Cheng et al.32 using specific antibodies to neutralize the coagulase in a mouse model, demonstrated that it is a critical virulence factor for the formation of abscesses and establishment of bacteremia. The authors suggested that the inhibition of coagulase could be useful against S. aureus infections.
Some studies shows that the decrease in the production of alpha-haemolysin can decrease the mortality of mice with S. aureus pneumonia. The smaller haemolysin production also minimizes the pathological characteristics of pulmonary injury, demonstrating the importance of studies that interfere on this virulence factor as a manner to minimize the infections.33,34
Hemolysin contribute to the damage of host cells membrane, causing its lysis, and subsequente to the invasion in adjacent tissue.35
However in the presente study it was not observed significant difference regarding the formation of haemolysin by S. aureus when in the presence of L. rhamnosus. Other works investigating the interaction between these microorganisms and the production of haemolysin for S. aureus were not found in the literature.
The present study demonstrated that L. rhamnosus also interfered significantly on the ability of biofilm formation by S. aureus. Similarly, Andre36 noted a decrease in biofilm formation, as well as interference in the composition of polysaccharides and DNA of S. aureus, by an antimicrobial peptide isolated from Lactococcus lactis. Zhou and Zang37 evaluated the action of bacteriocins produced by L. rhamnosus on biofilms of S. aureus developed on rabbits knee implants. The researchers obtained significant reduction of S. aureus on the implants treated with the bacteriocins, when compared to the control group.
Therefore, the results of the presente study showed inhibitory action of L. rhamnosus on the growth of S. aureus and on the expression of some virulence factors, suggesting a potential beneficial use of these probiotic bacteria in the prevention and/or treatment of staphylococcal diseases. However it is important to highlight that in vitro studies have limitations, since the cells are treated outside the “normal” environment. In vivo conditions include surrounding tissues, other microorganisms, blood supply, normal supply of nutrients, immune response and others. This way, further studies should be carried out, in order to confirm these possible benefits of L. rhamnosus and to define the best protocol for its use.
Conclusion
In conclusion, L. rhamnosus was able to inhibit S. aureus growth, as well as reduce the production of coagulase and the capacity of biofilm formation by these microorganism.
References
- Grundmann H, Aanensen DM, van den Wijngaard CC, et al. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular– pidemiological analysis. PLoS Med. 2010;7(1): e1000215.
- Ferreira MT, Manso AS, Gaspar P, et al. Effect of Oxygen on Glucose Metabolism: Utilization of Lactate in Staphylococcus aureus as Revealed by In Vivo NMR Studies. PLoS One. 2013;8(3):e58277.
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244–253.
- Prince T, McBain AJ, O’Neilla CA. Lactobacillus reuteri Protects Epidermal Keratinocytes from Staphylococcus aureus–Induced Cell Death by Competitive Exclusion. Appl Environ Microbio. 2012;78(15): 5119.
- Velazquez–Meza ME. Staphylococcus aureus methicillin–resistant: emergence and dissemination. Salud Pública Méx. 2005;47(5):381–387.
- Bonar E, Wójcik I, Wladyka B. Proteomics in studies of Staphylococcus aureus virulence. Acta Biochim Pol. 2015;62(3):367–381.
- Santos AL, Santos DO, Freitas CC, et al. Staphylococcus aureus: visitando uma cepa de importância hospitalar. J Bras Patol Med Lab. 2007;43(6):413–423.
- Crosby HA, Kwiecinski J, Horswill AR. Staphylococcus aureus aggregation and coagulation mechanisms, and their function in host–pathogen interactions. Adv Appl Microbiol. 2016;96:1–41.
- Kim H, Kim H S, Park WJ, et al. Inhibitory Effect of Lactobacillus plantarum Extracts on HT–29 Colon Cancer Cell Apoptosis Induced by Staphylococcus aureus and Its Alpha–Toxin. J Microbiol Biotechnol. 2015;25(11): 1849–1855.
- Even C, Marlière C, Ghigo JM, et al. Recent advances in studying single bacteria and biofilm mechanics. Adv Colloid Interface Sci. 2017;247:573–588.
- Huang R, Li M, Gregory RL. Bacterial interactions in dental biofilm. Virulence. 2011;2(5):435–444.
- Kiedrowski MR, Kavanaugh JS, Malone CL, et al. Nuclease Modulates Biofilm Formation in Community–Associated Methicillin–Resistant Staphylococcus aureus. PLoS One. 2011;6(11): e26714.
- Paharik AE, Horswill AR. The Staphylococcal Biofilm: Adhesins, regulation, and host response. Microbiol Spectr. 2015;4(2):10.
- Tagg JR, Dierksen KP. Bacterial replacement therapy: adapting “germ warfare” to infection prevention. Trends Biotechnol. 2003;21(5):217–223.
- Stanton TB. A call for antibiotic alternatives research. Trends Microbiol. 2013;21(3):111–113.
- Hauser AR, Mecsas J, Moir DT. Beyond antibiotics: new therapeutic approaches for bacterial infections. Clin Infect Dis. 2016;63(1):89–95.
- FAO/WHO, Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk With Live Lactic Acid Bacteria Cordoba. Argentina, 2001.
- Szajewska H. Probiotics and prebiotics in pediatrics: where are we now? Turk J Pediatr. 2007;49:231–244.
- Martín R, Miquel S, Ulmer J, et al. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microb Cell Fact. 2013;12:71.
- Malys MK, Campbell L, Malys N. Symbiotic and antibiotic interactions between gut commensal microbiota and host immune system. Medicina (Kaunas). 2015;51(2):69–75.
- Miettinen M, Pietilä TE, Kekkonen RA, et al. Nonpathogenic Lactobacillus rhamnosus activates the inflammasome and antiviral responses in human macrophages. Gut Microbes. 2012;3(6):510–522.
- Rocha–Ramírez LM, Pérez–Solano RA, Castañón–Alonso SL, et al. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J Immunol Res. 2017;4607491.
- Morais MB, Jacob CMA. O papel dos probióticos e prebióticos na prática pediátrica. J Pediatr. 2006;82(5): S189–S197.
- Lazarenko L, Babenko L, Sichel LS, et al. Antagonistic Action of Lactobacilli and Bifidobacteria in Relation to Staphylococcus aureus and Their Influence on the Immune Response in Cases of Intravaginal Staphylococcosis in Mice. Probiotics Antimicrob Proteins. 2012;4(2):78–89.
- Tagg JR, Dajami AS, Wannamaker LW. Bacteriocin of Gram positive bacteria. Bacteriol Rev. 1976;40(3):722–756.
- Bennett RW, Lancette GA. Staphylococcus aureus. In: Bacteriological Analytical Manual Online. 2001.
- Bertuccini L, Russo R, Iosi F, et al. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int J Immunopathol Pharmacol. 2017;30(2):163–167.
- Glück U, Gebbers J–O. Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and hemolytic streptococci). Am J Clin Nutr.2003; 77(2):517–520.
- Cui X, Shi Y, Gu S, et al. Antibacterial and antibiofilm activity of lactic acid bacteria isolated from traditional artisanal milk cheese from northeast China against enteropathogenic bacteria. Probiotics Antimicrob Proteins. 2018;10(4):601-610.
- Sadowska B, Walencka E, Wieckowska–Szakiel M, et al. Bacteria competing with the adhesion and biofilm formation by Staphylococcus aureus. Folia Microbiol (Praha). 2010;55(5):497–501.
- Subramanian A, Chitalia, VK, Bangera K, et al. Evaluation of HiaureusTMCoagulase Confirmation Kit in Identification of Staphylococcus aureus. J Clin Diagn Res. 2017;11(2):DC08-DC13.
- Cheng AG, McAdow M, Kim HK, et al. Contribution of Coagulases towards Staphylococcus aureus Disease and Protective Immunity. PLoS Pathogens. 2010;6(8):e1001036.
- Qiu J, Luo M, Wang J, et al. Isoalantolactone protects against Staphylococcus aureus pneumonia. FEMS Microbiol Lett .2011;324(2):147–155.
- Qiu J, Niu X, Dong J, et al. Baicalin Protects Mice From Staphylococcus aureus Pneumonia Via Inhibition of the Cytolytic Activity of α–Hemolysin. J Infect Dis. 2012;206(2):292–301.
- Seilie ES, Bubeck WJ. Staphylococcus aureus pore–forming toxins: The interface of pathogen and host complexity. Semin Cell Dev Biol. 2017;S1084–9521(17)30207–0.
- Andre C. Adesão. Formação e composição de biofilmes por Staphylococcus aureus em poliestireno na presença de nisina. Dissertação (mestrado) – Microbiologia Agrícola, Universidade Federal de Viçosa, 2015.
- Zhou B, Zhang D. Antibacterial effects of bacteriocins isolated from Lactobacillus rhamnosus (ATCC 53103) in a rabbit model of knee implant infection. Exp Ther Med. 2018;15(3):2985–2989.