Volume : 2 | Issue : 2
Case Report
Malignant transformation of proliferative verrucous leukoplakia to oral squamous cell carcinoma: Case report
Cintia Mussi Milani,1 Fernanda Vitória Guimarães,2 Natanael Henrique Ribeiro Mattos3
1Professor, Dentistry, Universidade Tuiuti do Paraná, Brazil
2Graduation student, Dentistry, Universidade Tuiuti do Paraná, Brazil
3Professor and Coordinator, Dentistry, Universidade Tuiuti do Paraná, Brazil
Received: March 25, 2019 | Published: April 18, 2019
Abstract
Objective: To report a case of proliferative verrucous leukoplakia (PVL) that progressed to squamous cell carcinoma in a 72-year-old female patient.
Case report: The patient was referred to a stomatologist's office for evaluation of diffuse whitish plaques, not rubbed off, with rough surfaces, suggestive of PVL. Histopathological examination of two lesions resulted in squamous cell carcinoma. The treatment included right mandibulectomy, left maxillectomy, bilateral cervical dissection and adjuvant treatment with chemotherapy and radiotherapy. The surgery was successful and until the present moment the patient is stable and under medical supervision.
Conclusion: PVL is a rare subtype of oral leukoplakia, with high recurrence rates and high risk of malignant transformation. Early diagnosis is the key word for a good prognosis for patients with PVL. In the present case, the late diagnosis and the progression to squamous cell carcinoma, resulted in an extensive surgery with high morbidity for the patient.
Keywords: Leukoplakia; proliferative verrucous leukoplakia; squamous cell carcinoma
Case report
A female patient with 72 years old was referred by her dental surgeon to a stomatologist in order to have some white lesions that she had in her mouth evaluated.
Anamnesis showed that she was systematically healthy and did not have any harmful habit. Patient complained that she's had pain in her mouth for 4 months and informed that she had already had a biopsy of the lower lip 6 years ago, with the diagnosis of lichen planus, which was being treated by another specialist.
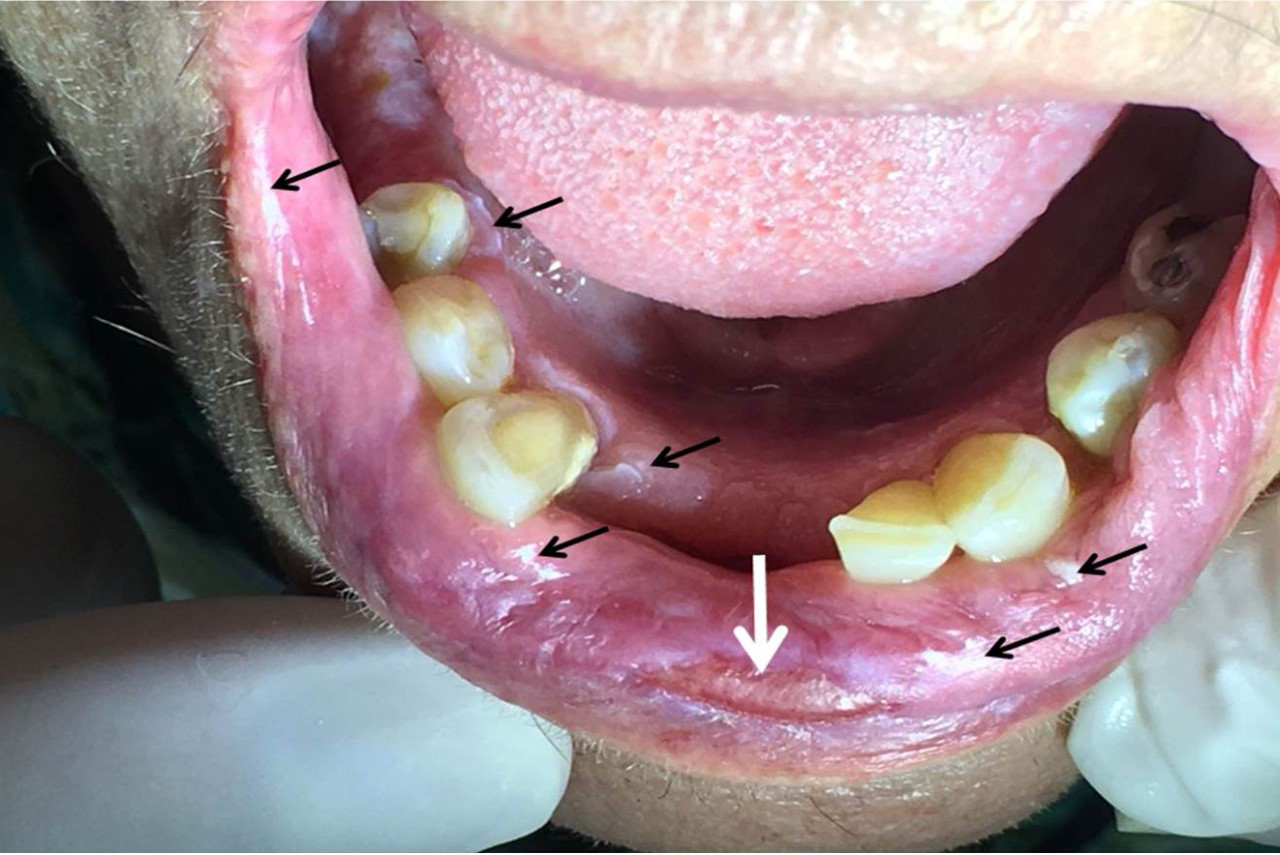
An extraoral physical exam did not show any facial asymmetry. During palpation of the lymph nodes, it was possible to observe a symptomatic mobile submental and submandibular chain with a volume increase. The lower lip mucosa presented a symptomatic flat ulcer in its central part with approximately 6 mm in length. Patient said that this ulcer sometimes was bigger and sometimes was smaller; totally disappearing for a couple of times (Figure 1).
|
Figure 1 Flat ulcer in lower labial mucosa (white arrow). We can also observe the presence of several leukoplastic plaques in this region, gingiva and alveolar ridge (black arrows). |
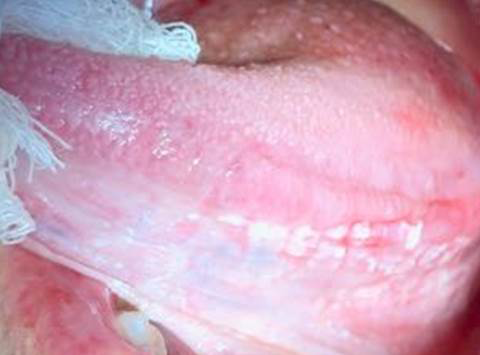
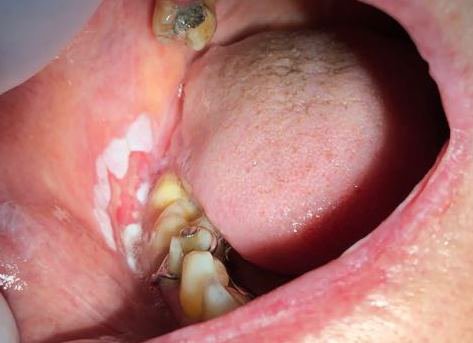
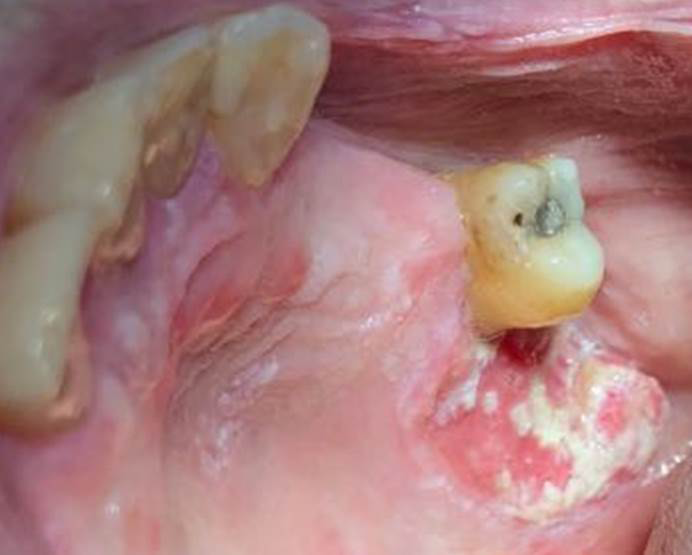
In the intraoral examination the presence of several white plaques was observed, with rough surfaces, that cannot be rubbed off, asymptomatic, in the region of attached gingiva, alveolar ridge, inferior surface of the tongue, hard palate and jugal mucosa. In this study, the presence of an extensive flat ulcer associated with the leukoplakia area was observed (Figures 2-4). In the left tuber, a mixed white/red lesion was observed, characteristic of speckled leukoplakia (Figure 5). When asked if she knew about these lesions, the patient reported that they were already present, in smaller size and quantity, at the time she did the biopsy of the lip, but the other professional had informed her that they were due to lichen planus and never told her about the need to have a biopsy. An initial diagnosis of PVL, with probable evolution to SCC, was established; the lip lesion suggested lichen planus of the erosive type.
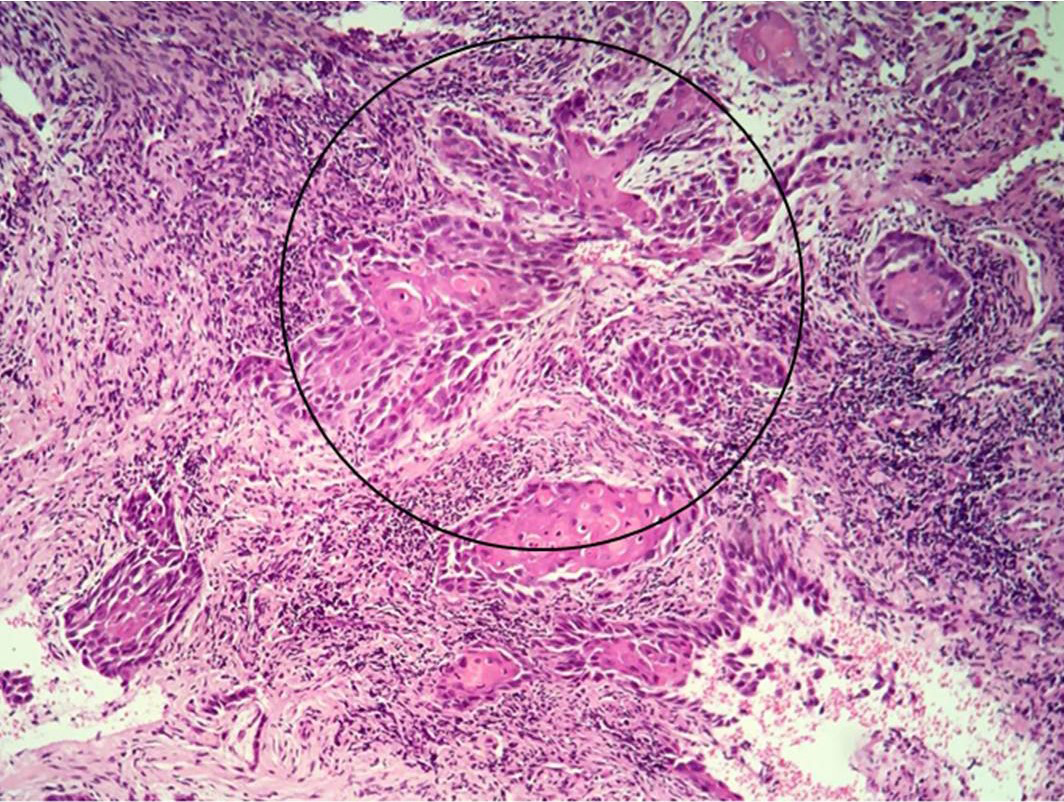
|
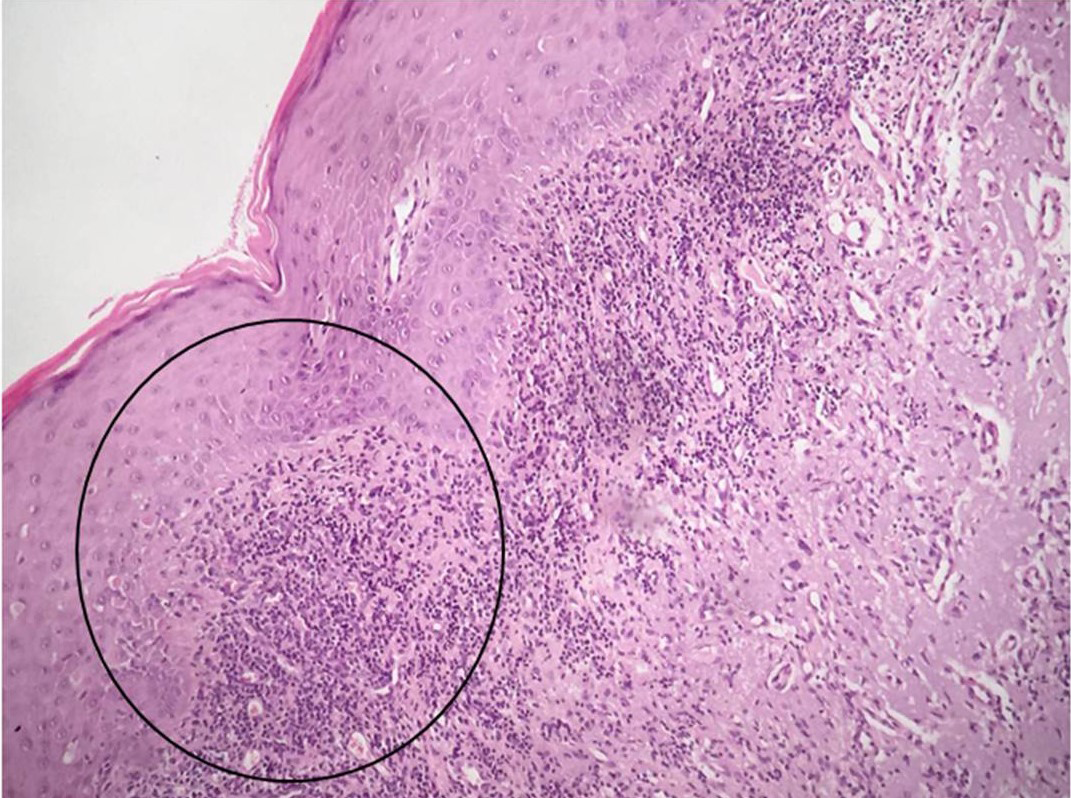
Figure 5 Histological aspect evidencing squamous cell blocks with nuclear atypia invading the superficial chorion. (Hematoxylin-eosin 100X). |
Preoperative tests (hemogram, coagulogram, blood glucose and creatinine) were requested, which were within the normal range. The incisional biopsy of the lesions in the left tuber, the right vestibule and the lower labial mucosa were performed with local anesthesia. The anatomopathological results were of SCC, SCC and lichen planus, respectively (Figures 6, 7).
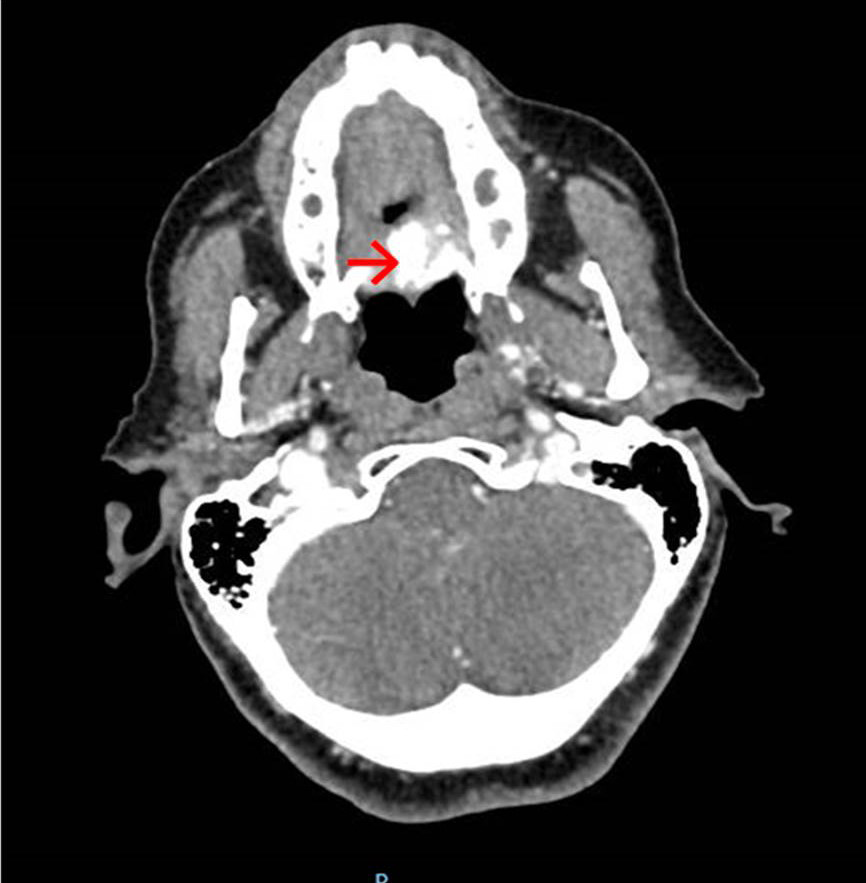
After the biopsy, the patient presented acute pain in the 27 region, which presented degree III mobility. As the pain did not subside with analgesic medication (tylex 30mg, every 6 hours, for 48 hours), its exodontia was performed. Since the preparation of the buccal cavity for head and neck radiotherapy was considered, the exodontia of the 17 and 37, which also presented great vertical bone loss, was performed at the same time. The patient was referred to the Head and Neck Surgery sector of Erasto Gaertner Hospital, where new biopsies were performed in the left palate and lower right alveolar ridge, and complementary examinations were requested: blood count, computed tomography of the neck and sinuses, electrocardiogram, pulmonary function test, and nutritionist evaluation. CT scan revealed erosion in the cortical bone of the alveolar recess of the left maxillary sinus, measuring approximately 19 x 14 x 10 mm in the largest diameters (Figure 8).
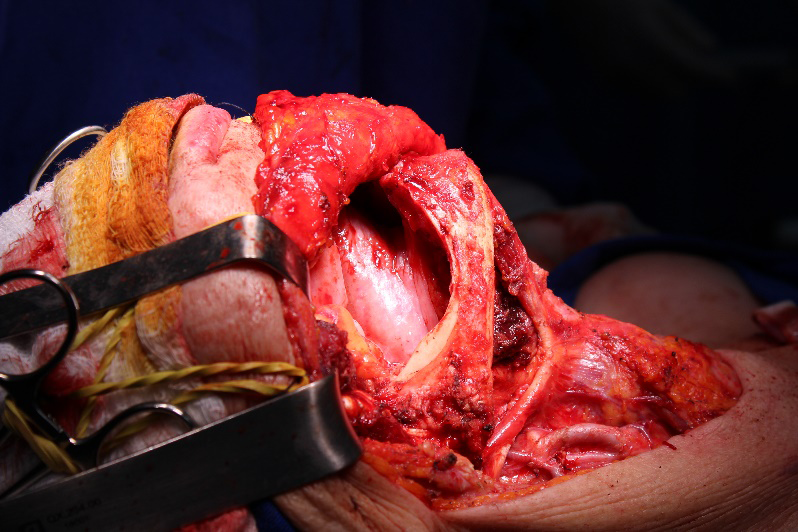
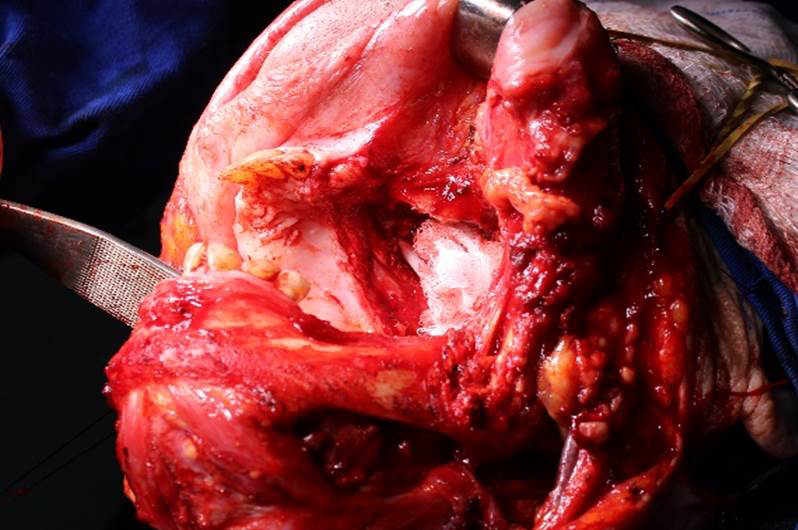
The patient was then referred to a head and neck, and plastic surgeons, who opted to initiate surgery, with bilateral cervical emptying, right side hemimandibulectomy, and left side maxillectomy (Figures 9,10). The plastic surgeons chose the right thigh as a graft donor area for the palate. An histopathological examination of the surgical specimens confirmed the diagnosis of SCC.
After surgery, the patient remained in the ICU for 1 day, being discharged 3 days later. Subsequently, she initiated the 2D EBRT radiotherapy (35 sessions) and CDDP 70mg/m2 chemotherapy, every 21 days (3 sessions). As a sequel to the adjuvant treatment, she presented trismus and oral mucositis.
Twelve months after surgery, the patient is stable, and maintains a semiannual follow-up with the oncological team.
Discussion
There are few studies on PVL and there are no consensus on the criteria of etiology and diagnosis.1,3 Its etiology remains obscure and some authors point out that, as in the present case, the use of tobacco and alcohol does not have the same influence on the appearance of this lesion as it does in other forms of leukoplakia and in SCC.7,9,10 Despite the association of human papillomavirus (HPV) with SCC, authors point out that in the case of PVL, this association cannot be established.2,6,9 Another difference between these two lesions is that unlike SCC, where men are most affected, PVL predominantly affects women, with some authors pointing to a ratio of 6:1; 2,5,7,8,10 in the study of Borgna et al6, however the woman/man ratio found was 1:1.
At an early stage, the clinical traits of PVL are indistinguishable from the other forms of leukoplakia.4,7 A homogeneous white plaque is observed which, over time, tends to recur and proliferate, becoming multifocal.5 The gum and jugal mucosa are the most affected sites, with less involvement of the tongue.2,3,5,9 Although lichen planus is part of the differential diagnosis of PVL and, in the present case, the patient presented erosive lichen type on the lip, intrabuccal lesions, characterized mainly by white multifocal plates, had no clinical manifestation that could be associated with it.
The clinical and histological evolution of the lesions is critical to obtain a correct diagnosis of the PVL, since there is no histopathological criterion specific for it. Suggestive signs of PVL include the presence of verrucous hyperplasia and various degrees of dysplasia.5 Multiple biopsies are required over time for comparison and histopathological evidence of lesion progression.5,9
The malignant transformation rate of PVL is high, affecting 48 to 71.2% of patients.5,6,10 Gouveia et al.10 point carcinogenesis as a result of an accumulation of genetic alterations that can lead to the instability of the chromosomes, in the form of numerical and structural aberrations, which can be detected as an abnormal DNA content or aneuploidy. Using cytometry and immunohistochemistry, the authors point out that the Mcm2 expression and DNA ploidy analysis can be used to predict areas of malignant transformation in patients with PVL.
Surgery, laser ablation, photodynamic therapy, retinoic acid, radiation and chemotherapy are not effective in reducing the recurrence and malignant transformation, emphasizing the resistance of PVL to various therapeutic modalities.3,9 Most patients undergo different treatments throughout the course of the disease, but surgical excision is the most used for it allows for a complete histological evaluation.5 For some authors, the most effective approach is to change the therapeutic goal of cure to control while maintaining a close surveillance to detect invasive diseases.4
It is important to emphasize the importance of the dental surgeon in performing an early diagnosis and rigorous follow-up, thus guaranteeing a more favorable prognosis for patients with this lesion. In the present case, late diagnosis, unfortunately, resulted in great patient morbidity.
References
- Cerero‒Lapiedra R, Baladé DM, Moreno López LA, et al. Proliferative verrucous leukoplakia: a proposal for diagnostic criteria. Med Oral Patol Oral Cir Bucal. 2010;15(5):276‒282.
- Upadhyaya JD, Fitzpatrick SG, Islam MN, et al. A Retrospective 20‒Year Analysis of Proliferative Verrucous Leukoplakia and Its Progression to Malignancy and Association with High‒risk Human Papillomavirus. Head and Neck Pathol. 2018;12(4):500‒510.
- Capella DL, Gonçalves JM, Abrantes AA, et al. Proliferative verrucous leukoplakia: diagnosis, management and current advances. Braz J Otorhinolaryngol. 2017;83(5):585‒593.
- Gillenwater AM, Vigneswaran N, Fatani H, et al. Proliferative Verrucous Leukoplakia (PVL): A Review of an Elusive Pathologic Entity!.Adv Anat Pathol. 2013; 20(6):416‒423.
- Abadie WM, Partington EJ, Fowler CB, et al. Optimal Management of Proliferative Verrucous Leukoplakia: A Systematic Review of the Literature. Otolaryngol Head Neck Surg. 2015;153(4):504‒511.
- Borgna SC, Clarke PT, Schache AG, et al. Management of proliferative verrucous leukoplakia: Justification for a conservative approach. Head Neck. 2017;39(10):1997‒2003.
- Munde A, Karle R. Proliferative verrucous leukoplakia: An update. J Cancer Res Ther. 2016; 12(2):469‒473.
- Richardson MS, Spruill L, Neville B. Proliferative Verrucous Leukoplakia: Review of an Evolving Entity. AJSP: Reviews & Reports. 2016; 21(3):132‒137.
- Staines K, Rogers H. Oral leukoplakia and proliferative verrucous leukoplakia: a review for dental practitioners. Br Dent J. 2017;223(9):665‒661.
- Gouvêa AG, Silva ARS, Speight PM, et al. High incidence of DNA ploidy abnormalities and increased Mcm2 expression may predict malignant change in oral proliferative verrucous leukoplakia. Histopathology. 2013; 62(4):551‒562.