Volume : 1 | Issue : 1
Technical Note
High-throughput microwell high-dimensional fluorescence activated Single cell sorting (HT-μW-HiD-FASCS)
Kartoosh Heydari
Director of Li Ka Shing Center FACS Core Facility, Department of Molecular Cell Biology / Cancer Research Laboratory, UC Berkeley, CA
Received: February 02, 2018 | Published:February 15, 2018
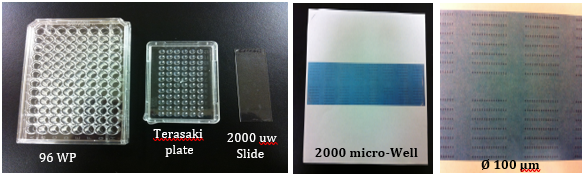
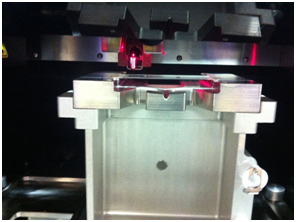
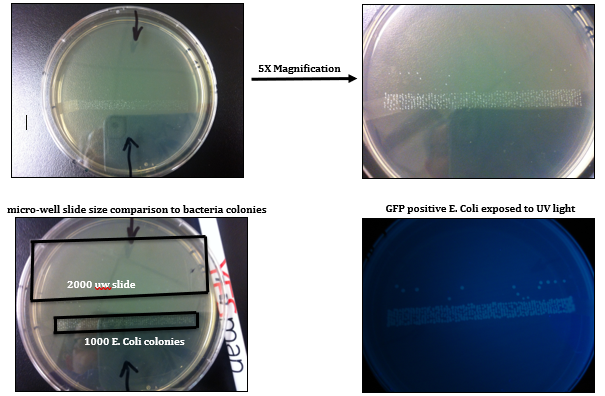
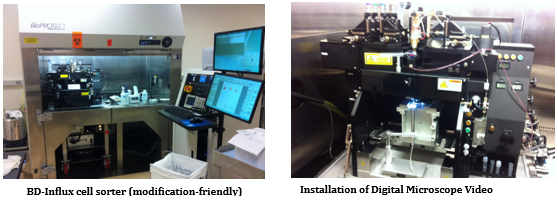
High-throughput screening (HTS) and isolation of single cell, in clonal level, for biochemical, genomic, epigenomic, transcriptomic, proteomic, or metabolomic studies has beenrecently addressed by many scientific communities in field of Cancer Immuno-biology/-therapy and Regenerative Medicine.Fluorescence Activated Cell Sorting (FACS) has been the most innovative technology in life sciences and cell therapy since its invention in 1969 by my former postdoctoral advisers at Stanford University, ProfessorL. A. Herzenbergs, who received the Kyoto prize (Japanese equivalent to Nobel Prize) in Advanced Technology in 2006. Using theinnovative capacity of High-DimensionalFACS (HiD-FACS) and HiD-Fluorescence Activated Single Cell Sorting (HiD-FASCS), we have added a novel concept in cell biology and regenerative medicine fields by describing a novel cell subset with unique function we named “Privileged Cells”1. We have now extended this innovative capacity of FASCS by {A} high-throughput(HT) single cell sorting (2000 wells/slid) in microWell (μW)(100 μm)(Figures 1–5 and Table 1), and {B} using high-resolution optical lens to improve the precision and the sensitivity of μW sort (Figure 4). HT-μW-HiD-FASCS is the most innovative technology for clonal level studies of the fine subsets of cells.
|
Figure 2: Development of High-Throughput (HT) microWell (μW) Fluorescence Activated Single Cell Sorting (FASCS). |
| Instrument setup | Sample Preparation |
|---|---|
|
|
Table 1: Key Criteria for a successful optimization of sort setting for HT-μW-HiD-FASCS.
References
- https://www.ipcc.ch/pdf/assessment-report/ar4/wg2/ar4_wg2_full_report.pdf
- http://pdf.wri.org/adapting_for_a_green_economy.pdf
- https://www.ipcc.ch/pdf/assessment-report/ar5/wg3/WGIIIAR5_SPM_TS_Volume.pdf
- https://www.cia.gov/library/publications/the-world-factbook/geos/pk.html
- http://www.prb.org/pdf11/2011population-data-sheet_eng.pdf
- http://www.maplecroft.com/about/news/natural_disasters.html
- http://siteresources.worldbank.org/SOUTHASIAEXT/Resources/Publications/448813-1231439344179/5726136-1259944769176/SAR_Climate_Change_Full_Report_November_2009.pdf
- http://onlinelibrary.wiley.com/doi/10.1002/wcc.26/abstract
- https://www.unisdr.org/files/657_lwr1.pdf
- http://www.prb.org/Educators/TeachersGuides/HumanPopulation/PopulationGrowth.aspx
- http://journals.sfu.ca/int_assess/index.php/iaj/article/viewFile/239/210
- https://germanwatch.org/klima/ccpi10.pdf
- http://pdf.usaid.gov/pdf_docs/PBAAB537.pdf
- http://www.unescap.org/stat/data/syb2009/ESCAP-SYB2009.pdf
- Bhandari Medani P. Environmental Performance and Vulnerability to Climate Change: A Case Study of India, Nepal, Bangladesh, and Pakistan. Climate Change and Disaster Risk Management. (2012);149−167.
- Bhandari Medani P, Bhattarai Keshav. Institutional Architecture for Sustainable Development: A Case Study from India, Nepal, Bangladesh, and Pakistan. ARMG Publishing. (2017);1(3):6−21.