Volume : 1 | Issue : 1
Review
Diagnostic approach & pharmacological treatment regimen of Peptic Ulcer Disease
Amrish Kumar, VrishDhwaj Ashwlayan, Mansi Verma
Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology, Dr. A. P. J. Abdul Kalam Technical University Lucknow, India
Received: August 22, 2018 | Published:January 01, 2019
Abstract
The Functional Assessment of Comfort Employing Technology Scale (FACETS) is introduced as an instrument that can be used with the general population to assess comfort utilizing different kinds of information technology (IT). In clinical settings, FACETS can inform individualized treatment planning, direct choice of media for communicating with each specific patient, and through improved communication, facilitate better treatment outcomes and higher satisfaction ratings. Scoring, interpretation, clinical application and implications of FACETS are described. FACETS was designed to be sensitive to numerous variables, including age, gender, and socio-economic status.
Keywords: Functional Assessment of Comfort Employing Technology Scale (FACETS); Treatment Planning; communication with patients; IT media
Abstract
Peptic ulcer is an acid related disorder of gastrointestinal tract. Gastritis, erosions, and peptic ulcer of the upper gastrointestinal (GI) tract require gastric acid for their formation. Peptic Ulcer Disease differs from gastritis and erosions in those ulcers typically extend deeper into the muscularis mucosa. There are three common forms of peptic ulcers, Helicobacter pylori associated, non-steroidal anti-inflammatory drug induced, and stress ulcers. The term “stress-related mucosal damage” is preferred to stress ulcer or stress gastritis, because the mucosal lesions range from superficial gastritis and erosions to deep ulcers. Chronic peptic ulcers vary in etiology, clinical presentation, and tendency to recur. HP-associated and NSAID induced ulcers develop most often in the stomach and duodenum of ambulatory patients. Occasionally, ulcers develop in the esophagus, jejunum, ileum, or colon. Peptic ulcers are also associated with Zollinger-Ellison syndrome (ZES), radiation, chemotherapy, and vascular insufficiency. In contrast, acute ulcers (Stress related mucosal disease) occur primarily in the stomach in critically ill hospitalized patient. This chapter focuses on chronic Peptic ulcer associated with Helicobacter pylori and NSAIDs. A brief discussion of ZES and upper Gastrointestinal tract bleeding related to Peptic ulcer and Stress related mucosal disease is included.
Keywords
peptic ulcer, gastrointestinal tract, zollinger-ellison syndrome, non-steroidal anti-inflammatory agents, stress related mucosal damage
Introductio
Ulcers are deep lesions penetrating through the entire thickness of the gastrointestinal tract mucosa and muscularis mucosa. Peptic ulcer has unquestionably been a disease of the twentieth century. Epidemiological data for this disease and its complications have shown striking geographical variations in incidence and prevalence. There are different types of ulcers most common are peptic ulcer, gastric ulcer, which appeared to be due to damage to the lining of the stomach, and duodenal ulcer, which was associated with excessive acid secretion by the stomach. The etiology of peptic ulcer was fiercely debated. It is believed that peptic ulcers develop due to an imbalance between aggressive factors (Helicobacter pylori, NSAIDs, gastric acid) and protective factors (mucin, bicarbonate, prostaglandins), leading to an interruption in the mucosal integrity.1 The natural course of chronic PUD is characterized by frequent ulcer recurrence. Approximately 60% to 100% of ulcers recur within1 year of initial ulcer healing with conventional antiulcer regimens. The most important factors that influence ulcer recurrence are HP infection and NSAID use. Other factors include gastric acid hyper-secretion, cigarette smoking, alcohol use, a long duration of PUD, ulcer-related complications, and patient non-compliance. Tobacco smoking delays healing of gastric ulcer and may influence duodenal ulceration. Seventy men, all cigarette smokers, were found to have duodenal ulceration at endoscopy. All were advised to stop smoking and received a three-month course of cimetidine. Endoscopy was repeated at three months (n = 63) and at six months (n = 56). At three months most (79%) patients showed ulcer healing and there was no difference between men who had and had not stopped smoking. At six months, however, a higher proportion (61% Vs 28%, p less than 0.05) of smokers (n = 38) than ex-smokers (n = 18) had duodenal ulceration. This difference reflected a combination of increased ulcer persistence and ulcer relapse. Neither cimetidine nor cigarette smoking nor ulcer healing appeared substantially to affect duodenitis and fixed deformity.2The cause of ulcer recurrence is most likely multifactorial. Table 1 revealed the comparison of common forms of peptic ulcer.
Characteristic |
H. pylori–induced |
NSAID-induced |
SRMD |
|---|---|---|---|
Condition |
Chronic |
Chronic |
Acute |
Site of damage |
Duodenum >stomach |
Stomach >duodenum |
Stomach >duodenum |
Intragastric pH |
More dependent |
Less dependent |
Less dependent |
Symptoms |
Usually epigastric pain |
Often asymptomatic |
Asymptomatic |
Ulcer depth |
Superficial |
Deep |
Most superficial |
GI bleeding |
Less severe, single vessel |
More severe, single vessel |
More severe, superficial mucosal capillaries |
Table 1 Comparison of common forms of peptic ulcer
Epidemiology
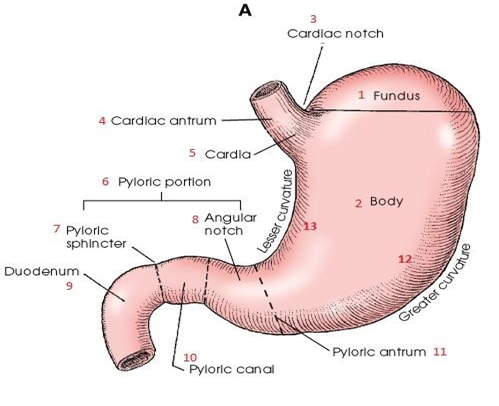
Approximately 10% of Americans develop chronic PUD during their lifetime. The incidence varies with ulcer type, age, gender, and geographic location. Race, occupation, genetic predisposition, and societal factors may play a minor role in ulcer pathogenesis but are attenuated by the importance of HP infection and NSAID use. The prevalence of PUD in the United States has shifted from predominance in men to nearly comparable prevalence in men and women. Recent trends suggest a declining rate for younger men and an increasing rate for older women. Factors that have influenced these trends include the declining smoking rates in younger men and the increased use of NSAIDs in older adults. Since 1960, ulcer-related physician visits, hospitalizations, operations, and deaths have declined in the United States by more than 50%, primarily because of decreased rates of PUD among men. The decline in hospitalizations has resulted from a reduction in hospital admissions for uncomplicated duodenal ulcer. However, hospitalizations of older adults for ulcer-related complications (bleeding and perforation) have increased.3Although the overall mortality from PUD has decreased; death rates have increased in patient’s older than 75 years of age, most likely a result of increased consumption of NSAIDs and an aging population. Patients with gastric ulcer have a higher mortality rate than those with duodenal ulcer because gastric ulcer is more prevalent in older individuals. Despite these trends remains one of the most common GI diseases, resulting in impaired quality of life, work loss, and high-cost medical care. To date, H2-receptor antagonists (H2RAs), proton pump inhibitors (PPIs), and drugs that promote mucosal defense have not altered PUD complication rates. Figure 1 represents the anatomical structure of the stomach’s regions.
Etiology and risk factors
Most peptic ulcers occur in the presence of acid and pepsin when HP (Helicobacter pylori), NSAIDs, or other factors (Table 2) disrupt normal mucosal defense and healing mechanisms. Hypersecretion of acid is the primary pathogenic mechanism in hypersecretory states such as ZES. Ulcer location is related to a number of etiologic factors. Benign gastric ulcers can occur anywhere in the stomach, although most are located on the lesser curvature, just distal to the junction of the antral and acid-secreting mucosa. Most duodenal ulcers occur in the first part of the duodenum (duodenal bulb).
Common causes |
|---|
Helicobacter pylori infection |
Nonsteroidal anti-inflammatory drugs |
Critical illness (stress-related mucosal damage) |
Uncommon causes |
Hypersecretion of gastric acid (e.g., Zollinger-Ellison syndrome) |
Viral infections (e.g., cytomegalovirus) |
Vascular insufficiency (crack cocaine–associated) |
Radiation |
Chemotherapy (e.g., hepatic artery infusions) |
Rare genetic subtypes |
Table 2 Potential causes of peptic ulcer
Helicobacter pylori
Helicobacter pylori infection causes chronic gastritis in all infected individuals and is causally linked to PUD, gastric cancer, and mucosa associated lymphoid tissue (MALT) lymphoma. However, only a small number of infected individuals will develop symptomatic PUD (about 20%) or gastric cancer (less than 1%). The pattern and distribution of gastritis correlates strongly with the risk of a specific gastrointestinal disorder. The development of atrophic gastritis and gastric cancer is a slow process that occurs over 20 to 40 years. Serologic studies confirm an association between HP and gastric cancer. Supportive evidence for PUD is based on the fact that most non-NSAID ulcers are infected with HP, and that HP eradication markedly decreases ulcer recurrence. Host-specific cofactors and HP strain variability play an important role in the pathogenesis of PUD and gastric cancer.4-6 Although an association between HP and PUD bleeding is less clear, eradication of HP decreases recurrent bleeding. No specific link has been established between HP and dyspepsia, nonulcer dyspepsia (NUD), or gastro-esophageal reflux disease.
Approximately 50% of the world’s population is colonized by HP. The prevalence of HP varies by geographic location, socioeconomic conditions, ethnicity, and age. In developing countries, HP prevalence exceeds 80% in adults and correlates with lower socioeconomic conditions. In industrialized countries, the prevalence of HP in adults is between 20% and 50%. The prevalence of HP in the United States is 30% to 40% but remains higher in ethnic groups such as African and Latin Americans. There is a decreasing frequency of infection, especially in regions with improving sanitation and socioeconomic conditions. In developed countries, there is an increased HP prevalence with age. However, this reflects a more intense transmission when older generations were children, as younger generations have been less likely to acquire the infection. Infection rates do not differ with gender or smoking status. HP is transmitted person-to-person by three different pathways: fecal-oral, oral-oral, and iatrogenic. Transmission of the organism is thought to occur by the fecal-oral route, either directly from an infected person, or indirectly from fecal-contaminated water or food. Members of the same household are likely to become infected when someone in the same household is infected. Risk factors include crowded living conditions, a large number of children, unclean water, and consumption of raw vegetables. Transmission by the oral-oral route has been postulated because HP has been isolated from the oral cavity. Transmission of HP can occur iatrogenically when infected instruments such as endoscopes are used.
Nonsteroidal Anti-Inflammatory Drugs
NSAIDs are one of the most widely prescribed classes of medications in the United States, particularly in individuals 60 years of age and older. There is overwhelming evidence linking chronic nonselective NSAID (including aspirin) use to a variety of GI tract injuries.7-9 Sub-epithelial gastric hemorrhages occur within 15 to30 minutes of ingestion, and progress to gastric erosions with continued ingestion. These lesions heal within a few days with continued NSAID use and do not lead to GI complications. Gastro-duodenal ulcers occur in 15% to 30% of regular NSAID users and may develop within a week or with continued treatment (6 months or longer). Gastric ulcers are most common, occur primarily in the antrum, and are of greater concern than erosions because of their potential to bleed or perforate. NSAID-induced ulcers may occur in the esophagus and colon but are less common. Each year, nonselective NSAIDs account for at least 16,500 deaths and 107,000 hospitalizations in the United States. Clinically important upper GI events occur in 3% to 4.5% of arthritis patients taking NSAIDs, and 1.5% have a serious complication (major GI bleeding, perforation, or obstruction). The risk factors for NSAID-induced ulcers and GI-related complications are presented in combinations of factors and confer an additive risk. The risk of NSAID complications is increased as much as 14-fold in patients with a previous history of an ulcer or ulcer-related bleeding. Advanced age is an independent risk factor and increases linearly with the age of the patient. The high incidence of ulcer complications in older individuals may be explained by age-related changes in gastric mucosal defense. The risk for NSAID-induced ulcers and complications is dose related, although both can occur with low dosages of nonprescription NSAIDs and the low dosages of aspirin taken for cardio-protective purposes (81 to 325 mg/day).10,11 The use of corticosteroids alone does not increase the risk of ulcer or complications, but ulcer risk is increased twofold in corticosteroid users who are also taking concurrent NSAIDs. The use of low-dose aspirin in combination with another NSAID increases the risk of upper GI complications to a greater extent than the use of either drug alone. The risk of bleeding is markedly increased when NSAIDs are used in combination with anticoagulants. NSAID-related dyspepsia that is not relieved by antiulcer medications may indicate an ulcer or ulcer complication, but dyspepsia does not correlate directly with mucosal injury or clinical events. Whether HP infection is a risk factor for NSAID-induced ulcers remains controversial.12-14 Most evidence indicates that both HP and NSAIDs are independent risk factors and that HP does not recent data suggest that HP may potentiate the effects of NSAIDs and low-dose aspirin with regard to ulcer bleeding. Cigarette smoking and alcohol ingestion contribute to increased ulcer risk but do not appear to be independent factors. There is little evidence to support clinically important differences with regard to the frequency of ulcers and upper GI complications among most available non-aspirin, nonselective NSAIDs when used in equipotent anti-inflammatory dosages. However, the nonacetylated salicylates (e.g., salsalate) and newer NSAIDs (e.g., etodolac, nabumetone, and meloxicam) may be associated with a decreased incidence of GI toxicity. NSAIDs that selectively inhibit cyclooxygenase-2 (COX-2) decrease the incidence of gastro-duodenal ulcers and related GI complications when compared to the nonselective NSAIDs. The use of buffered or enteric-coated aspirin confers no added protection from ulcer or GIcomplications.15-17
Cigarette smoking
There is epidemiologic evidence that links cigarette smoking to PUD, impaired ulcer healing, and ulcer-related GI complications. The risk is proportional to the number of cigarettes smoked and is modest when fewer than 10 cigarettes are smoked per day. Smoking does not increase ulcer recurrence after HP eradication. Death rates are higher among patients who smoke than among nonsmoking patients, although it is not known whether the increase in mortality reflects PUD or the cardiac and pulmonary sequelae of smoking. The exact mechanism by which cigarette smoking contributes to PUD remains unclear. Possible mechanisms include delayed gastric emptying of solids and liquids, inhibition of pancreatic bicarbonate secretion, promotion of duodeno-gastric reflux, and reduction in mucosal prostaglandin (PG) production. Although smoking increases gastric acid secretion, this effect is not consistent. It is uncertain whether nicotine or other components of smoke are responsible for these physiologic alterations. Cigarette smoking may provide a favorable milieu for HP infection.
Psychological stress
The importance of psychological factors in the pathogenesis of PUD remains controversial. Clinical observation suggests that ulcer patients are adversely affected by stressful life events. However, results from controlled trials are conflicting and have failed to document a cause-and-effect relationship. It is possible that emotional stress induced behavioral risks such as smoking and the use of NSAIDs, or alters the inflammatory response or resistance to HP infection. The role of stress and how it affects PUD is complex and probably multifactorial.
Dietary factors
The role of diet and nutrition in PUD is uncertain, but may explain regional variations. Coffee, tea, cola beverages, beer, milk, and spices may cause dyspepsia, but do not increase the risk for PUD. Beverage restrictions and bland diets do not alter the frequency of ulcer recurrence. Although caffeine is a gastric acid stimulant, constituents in decaffeinated coffee or tea, caffeine-free carbonated beverages, beer, and wine are also responsible for increasing gastric acid secretion. In high concentrations, alcohol ingestion is associated with acute gastric mucosal damage and upper GI bleeding; however, there is insufficient evidence to confirm that alcohol causes ulcers.
Diseases associated with peptic ulcers
There is epidemiologic evidence to suggest an increased prevalence of duodenal ulcers in patients with certain chronic diseases, but the pathophysiologic mechanisms of these associations are uncertain. A strong association exists in patients with systemic mastocytosis, multiple endocrine neoplasia type 1, chronic pulmonary diseases, chronic renal failure, kidney stones, hepatic cirrhosis, and α1-antitrypsindeficiency. An association may exist in patients with cystic fibrosis, chronic pancreatitis, Crohn’s disease, coronary artery disease, polycythemia vera, and hyperparathyroidism.
Pathophysiology
Gastric and duodenal ulcers occur because of an imbalance between aggressive factors (gastric acid and pepsin) and mechanisms that maintain mucosal integrity (mucosal defense and repair).
Gastric acid and pepsin
The potential for producing mucosal damage is related to the secretion of gastric (hydrochloric) acid and pepsin. Hydrochloric acid is secreted by the parietal cells, which contain receptors for histamine, gastrin, and acetylcholine. Acid (as well as HP infection and NSAID use) is an independent factor that contributes to the disruption of mucosal integrity. Increased acid secretion has been observed in patients with duodenal ulcers and may be a consequence of HP infection.18,19 Patients with ZES (described later in the chapter) have gastric acid hypersecretion resulting from a gastrin-producing tumor. Patients with gastric ulcer usually have normal or reduced rates of acid secretion (hypochlorhydria). Acid secretion is expressed as the amount of acid secreted under basal or fasting conditions, basal acid output (BAO); after maximal stimulation, maximal acid output (MAO); or in response to a meal. Basal, maximal, and meal-stimulated acid secretion varies according to time of day and the individual’s psychological state, age, gender, and health status. The BAO follows a circadian rhythm, with the highest acid secretion occurring at night and the lowest in the morning. An increase in the BAO: MAO ratio suggests a basal hypersecretory state such as ZES. A review of gastric acid secretion and its regulation can be found elsewhere. Pepsinogen, the inactive precursor of pepsin, is secreted by the chief cells located in the gastric fundus. Pepsin is activated by acid pH (optimal pH of 1.8 to 3.5), inactivated reversibly at pH 4, and irreversibly destroyed at pH 7. Pepsin appears to play a role in the proteolytic activity involved in ulcer formation.
Mucosal defense and repair
Mucosal defense and repair mechanisms protect the gastroduodenal mucosa from noxious endogenous and exogenous substances. Mucosal defense mechanisms include mucus and bicarbonate secretion, intrinsic epithelial cell defense, and mucosal blood flow. The viscous nature and near-neutral pH of the mucus-bicarbonate barrier protect the stomach from the acidic contents in the gastric lumen. Mucosal repair after injury is related to epithelial cell restitution, growth, and regeneration. The maintenance of mucosal integrity and repair is mediated by the production of endogenous prostaglandins. The term cytoprotection is often used to describe this process, but mucosal defense and mucosal protection are more accurate terms, as prostaglandins prevent deep mucosal injury and not superficial damage to individual cells. Gastric hyperemia and increased prostaglandin synthesis characterize adaptive cytoprotection, the short-term adaptation of mucosal cells to mild topical irritants. This phenomenon enables the stomach to initially withstand the damaging effects of irritants. Alterations in mucosal defense that are induced by HP or NSAIDs are the most important cofactors in the formation of peptic ulcers.
Helicobacter pylori
Helicobacter pylori is a spiral-shaped, pH-sensitive, gram-negative, micro aerophilic bacterium that resides between the mucus layer and surface epithelial cells in the stomach, or any location where gastric type epithelium is found. The combination of its spiral shape and flagellum permits it to move from the lumen of the stomach, where the pH is low, to the mucus layer, where the local pH is neutral. The acute infection is accompanied by transient hypochlorhydria, which permits the organism to survive in the acidic gastric juice. The exact method by which HP initially induces hypochlorhydria is unclear. One theory is that HP produces large amounts of urease, which hydrolyzes urea in the gastric juice and converts it to ammonia and carbon dioxide. The local buffering effect of ammonia creates a neutral microenvironment within and surrounding the bacterium, which protects it from the lethal effect of acid. HP also produces acid-inhibitory proteins, which allows it to adapt to the low-pH environment of the stomach. HP attaches to gastric-type epithelium by adherence pedestals, which prevent the organism from being shed during cell turnover and mucus secretion. Colonization of the corpus (body) of the stomach is associated with gastric ulcer. Antral organisms are hypothesized to colonize gastric metaplastic tissue (which is thought to arise secondary to changes in acid or bicarbonate secretion, products of HP, or host inflammatory responses) in the duodenal bulb, leading to duodenal ulcer. A number of bacterial and host factors contribute to the ability of HP to cause gastroduodenal mucosal injury. Pathogenic mechanisms include: (a) direct mucosal damage, (b) alterations in the host immune/inflammatory response, and (c) hypergastrinemia leading to increased acid secretion. In addition, HP enhances the carcinogenic conversion of susceptible gastric epithelial cells. Direct mucosal damage is produced by virulence factors (vacuolating cytotoxin, cytotoxin-associated gene protein, and growth inhibitory factor), elaborating bacterial enzymes (lipases, proteases, and urease), and adherence. About 50% of HP strains produce a protein toxin (Vac A) that is responsible for cellular vacuole formation. Strains with cytotoxin-associated gene (cagA) protein are associated with duodenal ulcer, atrophic gastritis, and gastric cancer. Lipases and proteases degrade gastric mucus, ammonia produced by urease may be toxic to gastric epithelial cells, and bacterial adherence enhances the uptake of toxins into gastric epithelial cells. HP infection alters the host inflammatory response and damages epithelial cells directly by cell-mediated immune mechanisms, or indirectly by activated neutrophils or macrophages attempting to phagocytose bacteria or bacterial products. HP infection may increase gastric acid secretion in patients with duodenal ulcer or diminish acid output in patients with gastric cancer. Antral-predominant infection is associated with hyper-gastrinemia and increased gastric acid secretion. Responsible mechanisms include cytokines, such as tumor necrosis factor-αreleased in HP gastritis; products of HP, such as ammonia; and diminished expression of somatostatin. Why somatostatin is diminished which is unclear, but cytokines may be involved. Corpus (body)-predominant infection promotes gastric atrophy and decreases acid output.
Nonsteroidal Anti-Inflammatory Drugs
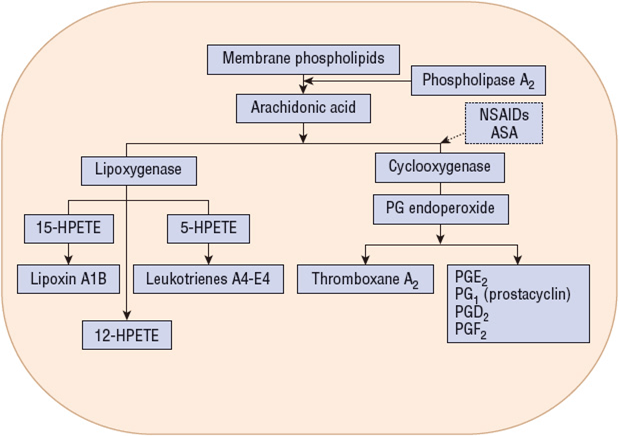
Nonselective NSAIDs including aspirin cause gastric mucosal damage by two important mechanisms: (a) direct or topical irritation of the gastric epithelium and (b) systemic inhibition of endogenous mucosal prostaglandin synthesis. Although the initial injury is initiated topically by the acidic properties of many of the NSAIDs, systemic inhibition of the protective prostaglandins plays the predominant role in the development of gastric ulcer. Cyclooxygenase (COX) is the rate-limiting enzyme in the conversion of arachidonic acid to prostaglandins and is inhibited by NSAIDs. Two similar COX isoforms have been identified: cyclooxygenase-1 (COX-1) is found in most body tissue, including the stomach, kidney, intestine, and platelets; cyclooxygenase-2 (COX-2) is undetectable in most tissues under normal physiological conditions, but its expression can be induced during acute inflammation and arthritis (Figure 2). COX-1 produces protective prostaglandins that regulate physiologic processes such as GI mucosal integrity, platelet homeostasis, and renal function. COX-2 is induced (upregulated) by inflammatory stimuli such as cytokines, and produces prostaglandins involved with inflammation, fever, and pain. COX-2 is also constitutionally expressed in organs such as the brain, kidney, and reproductive tract. Adverse effects (e.g. GI toxicity or renal toxicity) of NSAIDs are associated with the inhibition of COX-1, whereas anti-inflammatory actions result from NSAID inhibition of COX-2 Nonselective NSAIDs including aspirin inhibit both COX-1 and COX-2 to varying degrees. Aspirin irreversibly inhibits platelet COX-1 for as long as 18 hours, resulting in decreased platelet aggregation and prolonged bleeding times, which may potentiate upper and lower GI bleeding. Similar effects are observed with the nonselective NSAIDs. Figure 2 represents the metabolism of arachidonic acid after its release from membrane phospholipids and Figure 3 represents the tissue distribution and actions of cyclooxygenase (COX) isoenzymes.
Several other mechanisms may contribute to the development of NSAID-induced mucosal injury. Neutrophil adherence may damage the vascular endothelium and may lead to a reduction in mucosal blood flow or may liberate oxygen-derived free radicals and proteases. Leukotrienes, products of lipoxygenase metabolism, are inflammatory substances that may contribute to mucosal injury through stimulatory effects on neutrophil adherence (Figure 2). Topical irritant properties are predominantly associated with acidic NSAIDs (e.g., aspirin) and their ability to decrease the hydrophobicity of the mucous gel layer in the gastric mucosa. Most non-aspirin NSAIDs have topical irritant effects, but aspirin appears to be the most damaging. Although NSAID pro-drugs, enteric-coated aspirin tablets, salicylate derivatives, and parenteral or rectal preparations are associated with less-acute topical gastric mucosal injury, they can cause ulcers and related GI complications as a result of their systemic inhibition of endogenous PGs.
Diagnosis
Tests for Helicobacter pylori
The diagnosis of HP infection can be made using endoscopic or non-endoscopic tests (Table 3).20 The tests that require upper endoscopy are more expensive, uncomfortable, and require a mucosal biopsy for histology, culture, or detection of urease activity. Recommendations to maximize the diagnostic yield include taking at least three tissue samples from specific areas of the stomach, as patchy distribution of HP infection can lead to false-negative results. Because certain medications may decrease the sensitivity of these tests, antibiotics and bismuth salts should be withheld for 4 weeks and PPIs for 1 to 2 weeks prior to endoscopic testing. The non-endoscopic tests include serologic antibody detection tests, the urea breath test (UBT), and the stool antigen test. These tests are more convenient and less expensive than the endoscopic tests. Serologic tests are of limited use in evaluating post treatment eradication and are not reliable in young children. The UBT is based on HP urease activity. The 13-carbon (nonradioactive isotope) and 14-carbon (radioactive isotope) tests require that the patient ingest radiolabeled urea, which is then hydrolyzed by HP (if present in the stomach) to ammonia and radiolabeled bicarbonate. The radiolabeled bicarbonate is absorbed in the blood and excreted in the breath. A mass spectrometer is used to detect 13 carbon, whereas14carbon is measured using a scintillation counter. The stool antigen test is approved by the Food and Drug Administration (FDA), but availability in the United States is limited. It is less expensive and easier to perform than the UBT, and may be useful in children. Although comparable to the UBT in the initial detection of HP, the stool antigen test is less accurate when used to confirm HP eradicationpost-treatment.21 Salivary and urine antibody tests are under investigation. Testing for HP is only recommended if eradication therapy is considered. If endoscopy is not planned, serologic antibody testing is a reasonable choice to determine HP status. Post-treatment evaluation to confirm eradication is unnecessary in most patients with PUD unless they have recurrent symptoms, complicated ulcer, MALT lymphoma, or gastric cancer. The UBT is the preferred nonendoscopic method to verify HP eradication after treatment. To avoid confusing bacterial suppression with eradication, the UBT must be delayed at least 4 weeks after the completion of treatment. The term “eradication” or “cure” is used when post-treatment tests conducted 4 weeks after the end of treatment do not detect the organism. Quantitative antibody tests are considered impractical for post-treatment eradication as antibody titers remain elevated for long periods of time.
Test |
Description |
Comments |
|---|---|---|
Endoscopic tests |
||
Histology |
Microbiologic examination using various stains |
Gold standard; >95% sensitive and specific; permits classification of |
Culture |
Culture of biopsy |
Enables sensitivity testing to determine appropriate treatment or antibiotic |
Biopsy (rapid) urease |
HP urease generates ammonia, which |
Test of choice at endoscopy; >90% sensitive and specific; easily performed; |
Nonendoscopic tests |
||
Antibody detection |
Detects antibodies to HP in serum; in |
Quantitative; less sensitive and specific than endoscopic tests; more accurate |
Antibody detection (can be |
Detects lgG antibodies to HP in whole |
Qualitative; quick (within 15 minutes); unable to determine if antibody is |
Urea breath test |
HP urease breaks down ingested |
Tests for active HP infection; 95% sensitive and specific; results take about |
Stool antigen |
Identifies HP antigen in stool, leading |
Tests for active HP infection; sensitivity and specificity comparable to urea |
Table 3 Tests for detection of Helicobacter pylori
Imaging and endoscopy
The diagnosis of PUD depends on visualizing the ulcer crater either by upper GI radiography or endoscopy. Because of its lower cost, greater availability, and greater safety, many physicians believe that radiography should be the initial diagnostic procedure in patients with suspected uncomplicated PUD. If complications are thought to exist, or if an accurate diagnosis is warranted, upper endoscopy is the diagnostic procedure of choice. If a gastric ulcer is found on radiography, malignancy should be excluded by direct endoscopic visualization and histology.
Treatment
The treatment of chronic PUD varies depending on the etiology of the ulcer (HP or NSAID), whether the ulcer is initial or recurrent, and whether complications have occurred. Overall treatments aimed at relieving ulcer pain, healing the ulcer, preventing ulcer recurrence, and reducing ulcer-related complications. The goal of therapy in HP-positive patients with an active ulcer, a previously documented ulcer, or a history of an ulcer-related complication, is to eradicate HP, heal the ulcer, and cure the disease. Successful eradication heals ulcers and reduces the risk of recurrence to less than 10% at 1 year. The goal of therapy in a patient with a NSAID-induced ulcer is to heal the ulcer as rapidly as possible. Patients at high risk of developing NSAID ulcers should be switched to a COX-2 inhibitor or receive prophylactic drug co-therapy to reduce ulcer risk and ulcer-related complications. When possible, the most cost-effective drug regimen should be utilized.
General approach to treatment
The treatment of PUD centers on the eradication of HP in HP-positive patients and reducing the risk of NSAID-induced ulcers and ulcer related complications. Drug regimens containing antimicrobials such as clarithromycin, metronidazole, amoxicillin, and bismuth salts and anti-secretory drugs such as the PPIs or H2RAs are used to relieve ulcer symptoms, heal the ulcer, and eradicate HP infection. Successful eradication will alter the natural history of PUD and cure the disease. PPIs, H2RAs, and sucralfate are used to heal HP-negative NSAID-induced ulcers, but ulcer recurrence is likely in high-risk patients if the NSAID is continued. Prophylactic co-therapy with a PPI or misoprostol is used to decrease the risk of an ulcer and upper GI in patients taking nonselective NSAIDs. COX-2 inhibitors are often used in place of a nonselective NSAID to reduce the risk of ulcers and complications. Dietary modifications may be important for some patients, especially those who are unable to tolerate certain foods and beverages Lifestyle modifications such as reducing stress and decreasing or stopping cigarette smoking is often encouraged. Some patients may require radiographic or endoscopic procedures for a definitive diagnosis or for complications such as bleeding. Surgery may be necessary inpatients with ulcer-related bleeding or other complications such as perforation.
Nonpharmacologic therapy
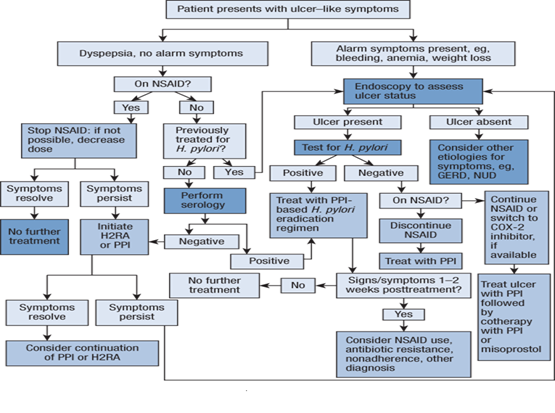
Patients with PUD should eliminate or reduce psychological stress, cigarette smoking, and the use of nonselective NSAIDs (including aspirin). Although there is no “antiulcer diet,” the patient should avoid foods and beverages (e.g., spicy foods, caffeine, and alcohol) that cause dyspepsia or that exacerbate ulcer symptoms. If possible, alternative agents such as acetaminophen, non-acetylated salicylate (e.g., salsalate), or COX-2 inhibitors should be used for relief of pain. Figure 4 shows an algorithm for guidelines for the evaluation and management of a patient who presents with dyspeptic or ulcer-like symptoms.
Elective surgery for PUD is rarely performed today because of highly effective medical management such as the eradication of HP and the use of potent acid inhibitors.22 A subset of patients, however, may require emergency surgery for bleeding, perforation, or obstruction. In the past, surgical procedures were performed for medical treatment failures and included vagotomy with pyloroplasty or vagotomy with antrectomy. Vagotomy (truncal, selective, or parietal cell) inhibits vagal stimulation of gastric acid. A truncal or selective vagotomy frequently results in postoperative gastric dysfunction and requires a pyloroplasty or antrectomy to facilitate gastric drainage. When an antrectomy is performed, the remaining stomach is anastomosed with the duodenum (Billroth I) or with the jejunum (Billroth II). A vagotomy is unnecessary when an antrectomy is performed for gastric ulcer. The postoperative consequences associated with these procedures include post vagotomy diarrhea, dumping syndrome, anemia, and recurrent ulceration.
Pharmacologic therapy
Treatment of Helicobacter pylori–associated ulcers. The goal of HP drug therapy is eradication of the organism. Treatment should be effective, well-tolerated, easy to comply with, and cost-effective. HP regimens should have eradication (cure)rates of at least 80% based on intention-to-treat analysis, or at least 90% based on per protocol analysis, and should minimize the potential for antimicrobial resistance.23,24 The use of a single antibiotic, bismuth salt, or antiulcer drug does not achieve this goal. However, clarithromycin is the single most effective antibiotic. Two-drug regimens that combine a PPI and either amoxicillin or clarithromycin have yielded marginal and variable eradication rates in the United States and are not recommended. In addition, the use of only one antibiotic associated with a higher rate of antimicrobial resistance. Eradication regimens that combine two antibiotics and one anti-secretory drug (triple therapy) or a bismuth salt, two antibiotics, and an antisecretory drug (quadruple therapy) increase eradication rates to an acceptable level and reduce the risk of antimicrobial resistance. When selecting a first-line eradication regimen, an antibiotic combination should be used that permits second-line treatment (if necessary) with different antibiotics. The antibiotics that have been most extensively studied and found to be effective in various combinations include clarithromycin, amoxicillin, metronidazole, and tetracycline. Although other antibiotics may be effective, they should not be used as part of the initial HP regimen. Because of insufficient data, ampicillin should not be substituted for amoxicillin, doxycycline should not be substituted for tetracycline, and azithromycin or erythromycin should not be substituted for clarithromycin. Amoxicillin should not be used in penicillin-allergic patients and metronidazole should be avoided if alcohol is consumed. Bismuth salts have a topical antimicrobial effect. Explanations as to why anti-secretory drugs enhance the efficacy of antibiotics include increased activity or stability of the antibiotic at a higher intra-gastric pH and enhanced topical antibiotic concentration resulting from decreased intra-gastric volume.
Proton pump inhibitor–based three-drug regimens
Proton pump inhibitor–based three-drug regimens with two antibiotics constitute first-line therapy for eradication of HP. A meta-analysis of 666 studies indicates that PPI-based regimens that combine clarithromycin and amoxicillin, clarithromycin and metronidazole, or amoxicillin and metronidazole yield similar eradication rates (78.9% to 82.8%) using intent-to-treat analysis; however, other studies suggest that the amoxicillin-metronidazole combinations less effective. Eradication rates were improved when the clarithromycin dose was increased to 1.5 g/day, but increasing the dosage of the other antibiotics did not increase eradication rates.25 Most clinicians prefer to initiate triple therapy with clarithromycin and amoxicillin rather than clarithromycin and metronidazole. Reserving metronidazole as an alternative or second-line agent leaves an effective back-up agent and reduces exposure and adverse effects from metronidazole. Alternatively, the PPI-clarithromycin-metronidazole regimen is an excellent alternative in penicillin-allergic patients. An initial 7-day course of therapy provides minimally acceptable eradication rates and has been approved by the FDA and is recommended in Europe. The duration of therapy, however, remains controversial in the United States, as longer treatment periods (10-day and 14-day) favor higher eradication rates and are less likely to be associated with antimicrobial resistance. One meta-analysis reports a 7% to 9% increase in eradication rates with a 14-day treatment regimen when compared to a 7-day regimen. A number of other antibiotics and antibiotic combinations have been evaluated as part of the PPI-based three-drug regimen with varying degrees of success. The PPI is an integral part of the three-drug regimen and should be taken 15 to 30 minutes before a meal (see section on PPIs) along with the two antibiotics. Although gastric acid inhibitions necessary to influence HP eradication rates, the specific level of inhibition remains unknown. A single daily dose of a PPI may be less effective than a double dose when used as part of a triple-therapy HP eradication regimen. Substitution of one PPI for another is acceptable and does not appear to enhance or diminish HP eradication. An H2RA should not be substituted for a PPI, as better eradication rates have been demonstrated with a PPI.26
Bismuth-based four-drug regimens
The bismuth-based four-drug regimens presented in were originally used as first-line therapy to eradicate HP. Eradication rates for a 14-day regimen containing bismuth, metronidazole, tetracycline, and H2-receptor antagonist are similar to those achieved with PPI-based triple therapy. Increasing the duration of treatment to1 month does not substantially increase eradication. Substitution of amoxicillin for tetracycline lowers the eradication rate and is usually not recommended. Substitution of clarithromycin 250 to 500 mg four times a day for tetracycline yields similar results, but increases adverse effects. The anti-secretory drug is also used to hasten pain relief in patients with an active ulcer. Although the original bismuth-based four-drug regimen is effective and inexpensive, it is associated with frequent adverse effects and poor compliance. A capsule containing bismuth, metronidazole, and tetracycline is under investigation. First-line treatment with quadruple therapy using a PPI (with bismuth, metronidazole, and tetracycline) in place of the H2RA achieves similar eradication rates as those of PPI-based triple therapy and permits a shorter treatment duration (7 days).27 Although evidence supports the efficacy of bismuth-based quadruple therapy as first-line treatment, it is often recommended as second-line treatment when a clarithromycin-amoxicillin regimen is used initially. All medications except the PPI (see section on PPIs) should be taken with meals and at bedtime.
Eradication regimens after initial treatment failure
HP eradication is often more difficult after initial treatment fails and eradication rates are extremely variable. Because there are limited data on second attempts to eradicate HP, treatment failures should be handled on a case-by-case basis. Failure of first- and second-line regimens in primary care requires referral to a specialist. Second-line empiric treatment should: utilize antibiotics that were not previously used during initial therapy; use antibiotics that do not have resistance problems; use a drug that has a topical effect such as bismuth; and the duration of treatment should be extended10 to 14 days. Thus after unsuccessful initial treatment with a PPI-amoxicillin-clarithromycin regimen, empiric second-line therapy should be instituted with bismuth subsalicylate, metronidazole, tetracycline, and a PPI for 10 to 14 days When metronidazole resistance is suspected, metronidazole may be replaced by furazolidone (100 mg four times a day) in either the proton pump inhibitor-based three-drug regimen or the bismuth-based four-drug regimen. When furazolidone is used, patients should be counseled not to ingest alcohol or monoamine oxidase inhibitors. Other successful second-line regimens are discussed elsewhere.28,29
Factors that contribute to unsuccessful eradication
Factors that contribute to unsuccessful eradication include poor patient compliance, resistant organisms, low intragastric pH, and a high bacterial load. Poor patient compliance is an important factor influencing successful therapy. Compliance decreases with multiple medications, increased frequency of administration, increased length of treatment, intolerable adverse effects, and costly drug regimens. Although longer treatment duration may contribute to noncompliance, missed doses in a 7-day regimen may also lead to failed eradication. Tolerability varies with different regimens. Metronidazole-containing regimens increase the frequency of adverse effects (especially when the dose is >1 g/day). Other common adverse effects include taste disturbances (metronidazole and clarithromycin), nausea, vomiting, abdominal pain, and diarrhea. Antibiotic-associated colitis, a serious complication, occurs occasionally. Oral thrush and vaginal candidiasis may also occur. An important determinant of successful HP eradication therapy is the presence of preexisting antimicrobial resistance. Metronidazole resistance is most common (10% to 60%) but varies depending on prior antibiotic exposure and geographic region. The clinical importance of metronidazole resistance in eradicating HP remains uncertain, as the synergistic effect of combining metronidazole with other antibiotics appears to render resistance to metronidazole less important. Primary resistance to clarithromycin is lower (10% to 15%) than with metronidazole, but it is more likely to affect the clinical outcome. Secondary resistance occurs in up to two thirds of treatment failures. Resistance to tetracycline and amoxicillin is uncommon. Resistance to bismuth has not been reported. The role of antibiotic sensitivity testing before initiating HP treatment has not been established. Table 4 revealed the oral drug regimens to cure peptic ulcer.
Treatment of NSAID-induced ulcers
Nonselective NSAIDs should be discontinued (when possible) if an active ulcer is confirmed. If the NSAID is stopped, most uncomplicated ulcers will heal with standard regimens of anH2-receptor antagonist, PPI, or sucralfate (Table 4). PPIs are usually preferred because they provide more rapid ulcer healing than H2RAs or sucralfate. If the NSAID must be continued in a patient despite ulceration, consideration should be given to reducing the NSAID dose, or acetaminophen, a nonacetylated salicylate, a partially selectiveCOX-2 inhibitor, or a selective COX-2 inhibitor. The PPIs are the drugs of choice when the NSAID must be continued, as potent acid suppression is required to accelerate ulcer healing.H2RAs are less effective in the presence of continued NSAID use; sucralfate does not appear to be effective. If HP is present, treatment should be initiated with an eradication regimen that contains a PPI.
Drug |
Duodenal or Gastric Ulcer Healing (mg/dose) |
Maintenance of Duodenal or Gastric Ulcer Healing (mg/dose) |
|---|---|---|
Proton pump inhibitors |
|
|
Omeprazole |
20–40 daily |
20–40 daily |
Lansoprazole |
15–30 daily |
15–30 daily |
Rabeprazole |
20 daily |
20 daily |
Pantoprazole |
40 daily |
40 daily |
Esomeprazole |
20–40 daily |
20–40 daily |
H2-receptor antagonists |
|
|
Cimetidine |
300 four times daily 800 at bedtime |
400–800 at bedtime |
Famotidine |
20 twice daily |
20–40 at bedtime |
Nizatidine |
150 twice daily |
150–300 at bedtime |
Ranitidine |
150 twice daily |
150–300 at bedtime |
Promote mucosal defense |
|
|
Sucralfate (g/dose) |
1 four times daily |
1–2 twice daily |
Table 4 Oral drug regimens used to heal peptic ulcers or maintain ulcer healing
Strategies to reduce the risk of NSAIDs-induced ulcers and ulcer-related Upper GI complication
A number of strategies are used to reduce the risk of NSAID-related ulcers and GI complications. Strategies aimed at reducing the topical irritant effects of nonselective NSAIDs prodrugs, slow-release formulations, and enteric-coated products—do not prevent ulcers or GI complications such as bleeding or perforation. Medical co-therapy with misoprostol or a PPI decreases the risk of ulcers and GI complications in high-risk patients Switching to a selective COX-2 inhibitor also decreases ulcer risk and complications.30-32
Misoprostol cotherapy with a nonselective NSAIDs
A multicenter, double-blind, placebo-controlled trial was undertaken to evaluate the efficacy of the synthetic prostaglandin E1 analog misoprostol in preventing and healing gastric ulcer induced by nonsteroidal anti-inflammatory drugs (NSAID) in patients receiving chronic NSAID therapy for osteoarthritis (OA). A total of 420 patients with OA and NSAID-associated abdominal pain who were receiving ibuprofen, piroxicam or naproxen were enrolled in the study. Endoscopy was performed at study entry and after 1, 2 and 3 months of continuous therapy with misoprostol 100 micrograms, misoprostol 200 micrograms or placebo given q.i.d. while NSAID therapy was continued. Treatment failure was defined as development of gastric ulcer (greater than 0.3 cm in diameter). The occurrence of ulcer in each misoprostol group (5.6% and 1.4% for 100 micrograms and 200 micrograms, respectively) was significantly lower (p less than 0.001) than that in the placebo group (21.7%). The statistically significant difference persisted when comparisons were restricted to development of ulcer greater than 0.5 cm in diameter (12.3, 4.2 and 0.7% for placebo, misoprostol 100 micrograms q.i.d. and misoprostol 200 micrograms q.i.d., respectively). Mild-to-moderate, self-limiting diarrhea was the most frequently reported adverse event attributed to misoprostol use.33
H2-receptor antagonist cotherapy with a nonselective NSAIDs
Standard H2-receptor antagonist dosages (e.g., famotidine 40 mg/day) are effective in reducing the risk of NSAID-induced duodenal ulcer, but not gastric ulcer (the most frequent type of ulcer associated with NSAIDs). Therefore, standard H2RA dosages should not be used as cotherapy with a nonselective NSAID for prophylaxis. There is evidence that higher dosages (e.g., famotidine 40 mg twice daily, ranitidine 300 mg twice daily) reduce the risk for gastric ulcer and
duodenal ulcer. However, there are no studies that have evaluated whether higher H2RA dosages reduce the risk of ulcer-related upper GI complications. The H2RAs may be used when necessary to relieve NSAID-related dyspepsia.
Proton pump inhibitor cotherapy with nonselective NSAIDs
Standard PPI dosages (e.g., omeprazole 20 mg/day and lansoprazole30 mg/day) reduce the risk of NSAID-induced gastric ulcer and duodenal ulcer. In a large comparative multicenter trial, omeprazole 20 mg/day was superior to ranitidine 150 mg twice daily in preventing NSAID-induced gastroduodenal ulcers. Two randomized controlled trials have compared with misoprostol and placebo. In the first study, omeprazole 20 mg/day was as effective as Misoprostol 400 mcg/day in reducing the incidence of gastric ulcer; however, if a higher dosage of misoprostol had been used it might have been more effective. In the second study of HP-negative NSAID users with a history of gastric ulcer, misoprostol 800 mcg/day was more effective than lansoprazole (15 mg or 30 mg/day) and placebo. When withdrawals from the study (primarily related to the side effects of misoprostol) were regarded as “treatment failures,” lansoprazole and full-dose misoprostol were considered clinically equivalent. Although there are no large clinical studies to prove that decrease the risk for NSAID-related upper GI complications, two small studies have reported a reduction in serious upper GI complications in patients with a history of upper GI bleeding.34,35 Proton Pump inhibitor cotherapy is considered an alternative to misoprostol in high-risk patients taking nonselective NSAIDs (including low-dose Aspirin).
Selective cox-2 inhibitors
Oral selective COX-2 inhibitors now available in the U.S. only. Celecoxib was investigated in arthritic patients in a large, long-term, randomized controlled trial (named CLASS) that was specifically designed to evaluate upper gastrointestinal complications versus nonselective NSAIDs.36,37 Patients in the CLASS trial, were permitted to take low-dose aspirin for cardio-protection. The initial analysis of the CLASS trial indicated that, when compared to nonselective NSAIDs, celecoxib had 50% fewer symptomatic ulcers and serious upper GI complications in patients not taking concomitant low-dose aspirin. However, in the CLASS trial, these benefits were negated in the aspirin users. Although a systematic review of celecoxib found that it is safer than nonselective NSAIDs, a re-evaluation of the CLASS data by the FDA concluded that celecoxib does not have a GI safety advantage over nonselective NSAIDs. The manufacturer of celecoxib argued (and the FDA acknowledged) that confounding factors in study design, including the use of low-dose aspirin, account for these discrepant results. Concerns about the cardiovascular safety of selective COX-2 inhibitors(e.g., thrombotic events and myocardial infarction) have arisen.GI effects such as dyspepsia and abdominal pain, fluid retention, hypertension, and renal toxicity can also occur with the COX-2inhibitors.Two small comparative trials in HP-negative patients with histories of NSAID-related ulcer complications suggested that a standard dosage of a PPI and a nonselective NSAID have a GI safety profile similar to that observed with a selective COX-2 inhibitor. However, the comparative benefits and cost effectiveness of these regimens remain controversial. Co-therapy with a PPI and a selective COX-2 inhibitor should be considered in patients with multiple or life-threatening risk factors.
Conventional treatment of active duodenal and gastric ulcers and long-term maintenance of ulcer healing
Conventional treatment with standard dosages of H2-receptor antagonists or sucralfate relieves ulcer symptoms and heals the majority of gastric and duodenal ulcers in 6 to 8 weeks.38 Proton pump inhibitors provide comparable ulcer healing rates over a shorter treatment period (4 weeks). A higher daily dose or a longer treatment duration is sometimes needed to heal larger gastric ulcers. Antacids, although effective, are not used as single agents to heal ulcers because of the high volume and frequent doses required (100 to 144 mEq of acid-neutralizing capacity 1 hour and 3 hours after meals and at bedtime), as well as associated adverse effects. When conventional antiulcer therapy is discontinued after ulcer healing, most HP-positive patients develop a recurrent ulcer within1 year. Continuous antiulcer therapy is aimed at the long-term maintenance of ulcer healing and at preventing ulcer-related complications. Because of HP eradication dramatically decreases ulcer recurrence (<10% at 1 year), continuous maintenance therapy has become largely obsolete. However, maintenance therapy may be indicated for patients who have a history of ulcer-related complications, a healed refractory ulcer, failed HP eradication therapy, or who are heavy smokers or NSAID users. Long-term maintenance therapy with an H2RA, PPI, or sucralfate is safe, but sucralfate should be avoided in renal impairment.
Treatment of refractory ulcers
Ulcers are considered refractory to therapy when symptoms, ulcers, or both persist beyond 8 weeks (duodenal ulcer) or 12 weeks (gastric ulcer) despite conventional treatment, or when several courses of HP eradication fail. Poor patient compliance, antimicrobial resistance, cigarette smoking, NSAID use, gastric acid hyper-secretion, or tolerance to the anti-secretory effects of an H2RA (see section on antiulcer agents) may contribute to refractory PUD. Patients with refractory ulcers should undergo upper endoscopy to confirm a non-healing ulcer, exclude malignancy, and assess HP status. HP-positive patients should receive eradication therapy (see section on treatment of HP associated ulcers). In HP-negative patients, higher PPI dosages (e.g., omeprazole 40 mg/day) heal the majority of ulcers. Continuous treatment with a PPI is often necessary to maintain healing, as refractory ulcers typically recur when therapy is discontinued, or the dose is reduced. Switching from one PPI to another is not beneficial. Patients with refractory gastric ulcer may require surgery because of the fear of malignancy.
Conclusion
The eradication of HP infection has dramatically changed the way in which chronic PUD is treated. Although substantial progress has been made, there is still no ideal treatment, and much of what has been learned has not yet been instilled into clinical practice. The widespread use of NSAIDs and their associated GI complications remains a major concern, especially in older adults. Co-therapy with misoprostol or a PPI, or switching to a selective COX-2 inhibitor reduces NSAID-related GI events, but studies are needed to determine their comparative cost effectiveness.
References
- Amandeep K, Robin S, Ramica S, Sunil K. Peptic ulcer: A review on etiology and pathogenesis. Int J Clin Pharm. 2012;3:34–38.
- Hull DH, Beale PJ. Cigarette smoking and duodenal ulcer. Gut. 1985;26(12):1333–1337.
- Del Valle J, Chey WD, Scheiman JM. Acid peptic disorders. In: Textbook of Gastroenterology. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2003:1321–1376.
- Suerbaum S, Michetti P. Helicobacter pylori infection. New England Journal of Medicine. 2002;347(15):1175–1186.
- Go MF. Natural history and epidemiology of Helicobacter pylori infection. Alimentary pharmacology & therapeutics. 2002;16:3–15.
- Peterson WL. Review article: Helicobacter pylori and gastric adenocarcinoma. Aliment PharmacolTher2002;16:(Suppl 1):40–46.
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001;120:594–606.
- Hawkey CJ. Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 2000;119(2):521–535.
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. New England Journal of Medicine. 1999;340(24):1888–1899.
- Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. Bmj. 2000;321(7270):1183–1187.
- Sørensen HT, Mellemkjaer L, Blot WJ, et al. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. The American journal of gastroenterology. 2000;95(9):2218–2224.
- Laine L. The effect of Helicobacter pylori infection on nonsteroidal anti-inflammatory drug-induced upper gastrointestinal tract injury. Alimentary pharmacology & therapeutics. 2002;16:34–39.
- Huang J-Q, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. The Lancet. 2002;359(9300):14–22.
- Lanas A, Fuentes J, Benito R, Serrano P, Bajador E, Sainz R. Helicobacter pylori increases the risk of upper gastrointestinal bleeding in patients taking low-dose aspirin. Alimentary pharmacology & therapeutics. 2002;16(4):779–786.
- Micklewright R, Lane S, Linley W, McQuade C, Thompson F, Maskrey N. NSAIDs, gastroprotection and cyclo-oxygenase-II-selective inhibitors. Alimentary pharmacology & therapeutics. 2003;17(3):321–332.
- Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proceedings of the National Academy of Sciences. 1999;96(13):7563–7568.
- Kelly JP, Kaufman DW, Jurgelon JM, Sheehan J, Koff RS, Shapiro S. Risk of aspirin-associated major upper-gastrointestinal bleeding with enteric-coated or buffered product. The Lancet. 1996;348(9039):1413–1416.
- Del Valle J, Todisco A. Gastric secretion. In: Textbook of Gastroenterology. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2003:266–307.
- Sachs G, Shin JM, Munson K, et al. The control of gastric acid and Helicobacter pylori eradication. Alimentary pharmacology & therapeutics. 2000;14(11):1383–1401.
- Vaira D, Gatta L, Ricci C, Miglioli M. Diagnosis of Helicobacter pylori infection. Alimentary pharmacology & therapeutics. 2002;16:16–23.
- Bilardi C, Biagini R, Dulbecco P, et al. Stool antigen assay (HpSA) is less reliable than urea breath test for post-treatment diagnosis of Helicobacter pylori infection. Alimentary pharmacology & therapeutics. 2002;16(10):1733–1738.
- Seymour M, Andersen D. Surgery for Peptic Ulcer Disease and postgastrectomy syndromes. In: Textbook of Gastroenterology. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2003:1441–1454.
- Malfertheiner P, Mégraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection—The Maastricht 2-2000 Consensus Report. Alimentary pharmacology & therapeutics. 2002;16(2):167–180.
- Qasim A, O’morain CA. Treatment of Helicobacter pylori infection and factors influencing eradication. Alimentary pharmacology & therapeutics. 2002;16:24–30.
- Rossum LV. Evaluation of treatment regimens to cure Helicobacter pylori infection—a meta-analysis. Alimentary pharmacology & therapeutics. 1999;13(7):857–864.
- Gisbert JP, Khorrami S, Calvet X, Gabriel R, Carballo F, Pajares JM. Meta-analysis: proton pump inhibitors vs. H2-receptor antagonists—their efficacy with antibiotics in Helicobacter pylori eradication. Alimentary pharmacology & therapeutics. 2003;18(8):757–766.
- Gene E, Calvet X, Azagra R, Gisbert JP. Triple vs. quadruple therapy for treating Helicobacter pylori infection: a meta-analysis. Alimentary pharmacology & therapeutics. 2003;17(9):1137–1143.
- Megraud F, Lamouliatte H. the treatment of refractory Helicobacter pylori infection. Alimentary pharmacology & therapeutics. 2003;17(11):1333–1343.
- Gisbert JP, Pajares JM. Helicobacter pylori ‘rescue’ regimen when proton pump inhibitor-based triple therapies fail. Alimentary pharmacology & therapeutics. 2002;16(6):1047–1057.
- Chan FKL, Leung WK. Peptic-ulcer disease. Lancet. 2002;360(9337):933-941.
- Fennerty MB. NSAID-related gastrointestinal injury: Evidence-based approach to a preventable complication. Postgraduate Medicine. 2001;110(3):87-94. doi:10.3810/pgm.2001.09.1020
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. New England Journal of Medicine. 2001;345(6):433–442.
- Roth SH. Misoprostol in the prevention of NSAID-induced gastric ulcer: a multicenter, double-blind, placebo-controlled trial. The Journal of rheumatology supplement. 1990;20:20–24.
- Chan FK, Chung SS, Suen BY, et al. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. New England journal of medicine. 2001;344(13):967–973.
- Lai KC, Lam SK, Chu KM, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. New England Journal of Medicine. 2002;346(26):2033–2038.
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Jama. 2000;284(10):1247–1255.
- Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. New England Journal of Medicine. 2000;343(21):1520–1528.
- Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. Journal of the American Pharmaceutical Association. 2000;40(1):52–62.