Volume : 1 | Issue : 1
Review
Cost-effectiveness of Sorafenib for hepatocellular carcinoma: a systematic review
Mohammad Hossein Motevalli, Farzad Peiravian, Saeed Taheri
Department of Pharmacoeconomics and Pharma Management, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Received: September 26, 2018 | Published:January 02, 2019
Abstract
Background: Hepatocellular carcinoma (HCC) is the sixth common cancer (5th common cancer in men and 7th common cancer in women) worldwide. In patients who have advanced HCC, sorafenib is the first-line of treatment and showed an improvement of 37.3% in patients’ overall survival. This study was aimed to systematically review the economic evaluations of sorafenib in the treatment of HCC to assist decision-makers in controlling costs and ensuring access to effective treatments within limited healthcare budgets.
Method: A systematic review was conducted in major databases including PubMed, Cochrane, Embase, Google scholar, Scopus, and Web of Science up to August 2018 with limitation to English full-text published articles. Articles were selected on the basis of their dealing with the economic evaluations of sorafenib. The quality of the selected studies was assessed using the Quality of Health Economic Studies (QHES) instrument.
Results: 13 articles were identified in databases meeting the inclusion/exclusion criteria. Five of them had compared sorafenib versus best supportive care (BSC). The others had compared sorafenib versus different treatment strategies such as oxaliplatin plus infusional-fluorouracil/leucovorin (FOLFOX4), transarterial radioembolization (TARE), transarterial chemoembolization (TACE), and etc. This review found incremental costs per quality-adjusted life years in the range of US$ -1,014,507 to US$ 934,803, depending on whether the setting was considered and which comparator was utilized. In all cases, sorafenib was associated with higher cost and higher efficacy except in comparison with TACE and TARE. Sorafenib was found as a dominant and dominated strategy versus TACE and TARE, respectively.
Conclusion: This review revealed that sorafenib was more effective with higher cost than its comparators except TARE and could be considered as a cost-effective treatment option for patients with metastatic HCC. The results should be interpreted with a view to the specific health care settings and willingness to pay threshold in different jurisdictions.
Keywords: hepatocellular carcinoma, pharmacoeconomic evaluation, sorafenib, cost-effectiveness, systematic review
Introduction
Hepatocellular carcinoma (HCC) includes 85-90% of primary liver cancer. HCC is the sixth common cancer: 5th common cancer in men and 7th common cancer in women worldwide. In addition, the third reason of the cancer-related death after lung and stomach cancers is HCC (4).1 The majority of HCC incidence are in East Asia and sub-Saharan Africa. The overall 5-year cost of care for HCC patients was approximately $106.4 million in Canada, in 2009.2 20.9 million Disability adjusted life year (DALYs) was the result of liver cancer in 20133. Disease is associated with a poor prognosis and just 6.9% of patients have 5-year survival mainly because a small group of patients are diagnosed in early stages2. Hepatitis B virus, hepatitis C virus, cirrhosis, environmental toxins such as aflatoxin, alcohol abuse, tobacco, type 2 diabetes mellitus, obesity are the most important risk factors of HCC.4
There are different approved treatments based on disease stage such as Stereotactic body radiotherapy (SBRT), transarterial radioembolization (TARE) transarterial chemoembolization (TACE), liver transplantation (LT), radiofrequency ablation(RFA), microwave ablation, percutaneous ethanol or acetic acid ablation, cryoablation, systemic chemotherapy, and molecularly targeted therapies such as doxorubicin for hepatocellular carcinoma.1 In patients who have advanced HCC, sorafenib is recommended as the first-line of treatment in different guidelines.5–7 Sorafenib were helped to improve overall survival around 37.3%.8
Sorafenib is a multi-kinase inhibitor such as vascular endothelial growth factor receptor (VEGFR) that decreases tumor cell proliferation. Sorafenib was approved in 2007 for hepatocellular carcinoma as an oral medication administrated 400mg twice daily. However, according to recent studies, dose-adjusted sorafenib regimen results in better efficacy-safety balanced.9
In light of the ever-increasing costs of the healthcare system and a great expense of the newly introduced treatments, it has become more crucial than ever before to choose more cost-effective treatments among different alternatives. Cost-effectiveness studies may provide healthcare decision-makers with the requisite insights to make informed choices.
Hence, this study was aimed to systematically review the economic evaluations of SFN in the treatment of HCC to assist decision-makers in controlling costs and ensuring access to effective treatments within limited healthcare budgets.
Methods
Literature search strategyA systematic literature search in EMBASE, MEDLINE, PUBMED, Google Scholar, and the Scopus database was conducted between November 2004 and August 2018. Table 1 shows key words which have been used in our search strategy.
Keywords |
1. Sorafenib |
8. HCC |
|---|---|---|
Search strategy |
(1 OR 2) AND (((3 OR 4) AND (5 OR 6)) OR 7 OR 8) AND (11 OR 12 OR 13 OR 14) |
|
Table 1 Search strategy and keywords in which used
The search was limited to articles published in English. In addition, studies were omitted if the full-text was not available. The included studies should also meet the following inclusion criteria:
- Adult with intermediate or advanced hepatocellular carcinoma as patient population.
- Taking into account both cost and clinical consequences.
- Representing sorafenib-containing therapy as one of the treatments arm
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was used to carry out the systematic review.10 Then, selected articles were transferred to the Mendeley reference manager software and duplicated documents found in different databases were counted out. Then, the full text of the articles were screened by two reviewers ensure compliance with the inclusion and exclusion criteria and, finally, the data from eligible studies were extracted onto an Excel spreadsheet. In case of disagreement between researchers, a third reviewer decided on discrepancies of the articles.
To evaluate the quality of the included studies, the Quality of Health Economic Studies (QHES) instrument, which is shown in Table 2, was used. QHES score shows the quality of economic studies as follows: poor (QHES score<50), fair (QHES score ≥ 50 and <75), and good (QHES score ≥75 and ≤100).11
Questions |
Points |
|
|---|---|---|
1. |
Was the study objective presented in a clear, specific, and measurable manner? |
7 |
2. |
Were the perspective of the analysis (societal, third-party payer, etc.) and reasons for its selection stated? |
4 |
3. |
Were variable estimates used in the analysis from the best available sources (i.e., randomized control trial - best, expert opinion - worst)? |
8 |
4. |
If estimates came from a subgroup analysis, were the groups pre-specified at the beginning of the study? |
1 |
5. |
Was uncertainty handled by (1) statistical analysis to address random events, (2) sensitivity analysis to cover a range of assumptions? |
9 |
6. |
Was incremental analysis performed between alternatives for resources and costs? |
6 |
7. |
Was the methodology for data abstraction (including the value of health states and other benefits) stated? |
5 |
8. |
Did the analytic horizon allow time for all relevant and important outcomes? Were benefits and costs that went beyond 1 year discounted (3% to 5%) and justification given for the discount rate? |
7 |
9. |
Was the measurement of costs appropriate and the methodology for the estimation of quantities and unit costs clearly described? |
8 |
10. |
Were the primary outcome measure(s) for the economic evaluation clearly stated and did they include the major short-term, long-term, and negative outcomes? |
6 |
11. |
Were the health outcomes measures/scales valid and reliable? If previously tested valid and reliable measures were not available, was justification given for the measures/scales used? |
7 |
12. |
Were the economic model (including structure), study methods and analysis, and the components of the numerator and denominator displayed in a clear and transparent manner? |
8 |
13. |
Were the choice of economic model, main assumptions, and limitations of the study stated and justified? |
7 |
14. |
Did the author(s) explicitly discuss the direction and magnitude of potential biases? |
6 |
15. |
Were the conclusions/recommendations of the study justified and based on the study results? |
8 |
16. |
Was there a statement disclosing the source of funding for the study? |
3 |
Total Points |
100 |
|
Table 2 The Quality of Health Economics Studies (QHES) instrument
Results
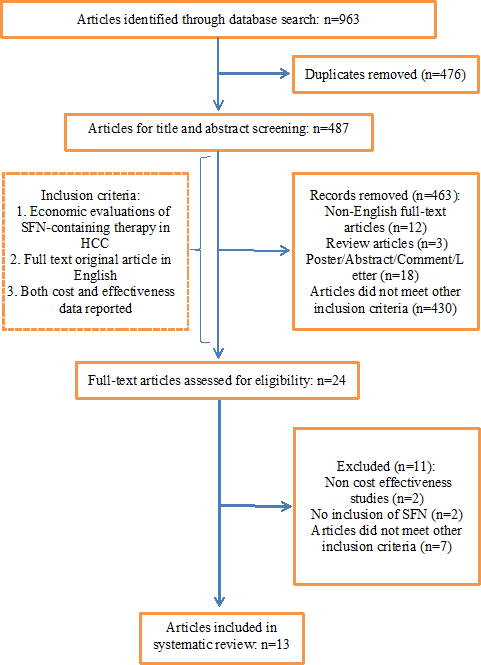
OverviewIn this comprehensive systematic review 487 articles were identified. After primary evaluation by screening the titles and abstracts, according to the inclusion criteria, 24 articles were selected for full-text evaluation. Finally, 13 articles were selected for final analysis whose characteristics have been presented in Tables 3 and 4. Six of the selected studies had compared sorafenib versus best supportive care (BSC). Other studies had compared sorafenib versus SBRT, TACE, FOLFOX4, TARE, and other therapies. The PRISMA flow chart of this study is given in Figure 1.
Study |
Country |
Perspective |
Currency/year |
Economic model type |
Time horizon |
Discount rate |
Funding source |
|---|---|---|---|---|---|---|---|
Muszbek et al. (2008) 12 |
Canada |
Canadian provincial Ministry of Health |
Canadian dollars/ 2007 |
Markov |
Lifetime |
5% |
Bayer HealthCare Pharmaceuticals |
Vitale et al. (2010) 13 |
Italy |
Payer |
Euro/ 2008 |
Markov |
10 year |
3% |
Not reported |
Carr et al. (2010)14 |
USA |
Third-party-payer |
Dollars/ 2007 |
Markov |
Lifetime |
3% |
Bayer HealthCare Pharmaceuticals |
Cammà et al. (2013)15 |
Italy |
Third-party managed-care payer |
Euro/ 2012 |
Markov |
5 year |
3% |
Not reported |
Zhang et al. (2015)16 |
China |
Chinese payer |
Dollars/ |
Markov |
NA |
3% |
No |
Zhang et al. (2016)17 |
China |
Chinese societal |
Dollars/NA |
Markov/ cohort |
10 year |
Not reported |
No |
Leung et al. (2016)18 |
Taiwan |
National Health Insurance |
NT$/2015 |
Markov |
5 year |
3% |
No |
Rognoni et al. (2017)19 |
Italy |
Healthcare system |
Euro/2016 |
Markov |
Lifetime |
3.5% |
ASBM Srl through an unrestricted grant to CERGAS, Bocconi University |
Parikh et al. (2017)20 |
USA |
Medicare |
Dollars/ 2015 |
NA |
2 year |
NA |
NOT reported |
Zhao et al. (2017)21 |
China |
Healthcare system |
Dollars/ 2015 |
Markov/ cohort |
Lifetime |
3% |
No |
Chen et al. (2018)9 |
China/ USA |
Healthcare system |
Dollars/ 2016 |
Markov/ cohort |
2 year |
3% |
National Natural Science Foundation of China and Pearl River S&T Nova Program of Guangzhou, China |
Ho et al. (2018)22 |
Taiwan |
Healthcare payer |
NT$/2014 |
Markov |
5 year |
3% |
China Medical University grant |
Qin et al. (2018)23 |
China |
Healthcare system/patients |
Dollars/ 2017 |
Markov |
Lifetime |
5% |
Sanofi china |
Table 3 Characteristics of articles assessing cost-effectiveness of Sorafenib in hepatocellular carcinoma
|
Study |
Population |
Comparator |
Type of cost included |
Cost |
Effectiveness |
ICER |
|---|---|---|---|---|---|---|
|
Muszbek et al. (2008) 12 |
Advanced HCC |
SFN vs BSC |
Hospitalization, Medical staff, visits Lab tests,AE, drug and supportive care |
Can$47,272 vs Can$10,309 |
LYs: 1.51 vs 1.02 |
Can$ 75,759/LYG |
|
Vitale et al. (2010) 13 |
Waiting list for liver transplantation |
SFN+LT vs LT |
Sorafenib, Percutaneous ablation, Chemoembolization, Follow-up, Transplantation, indirect cost, drugs |
NA |
QALD:1350 vs 1244 |
€197/QALD |
|
Carr et al. (2010)14 |
Advanced HCC |
SFN vs BSC |
Drugs, physician visits, laboratory tests, scans, and hospitalizations |
$US40,639 vs $US7,804 |
LYs: 1.58 vs 1.05 |
$62,473/LYG |
|
Cammà et al. (2013)15 |
Intermediate and Advanced HCC |
SFN full dose vs BSC |
Drugs, diagnostic exams, visits, hospitalization |
€16,081 vs 4,142 |
QALY: 0.16 vs 0.012 |
€69,344/QALY |
|
SFN adjusted dose vs BSC |
€19,944 vs 4,142 |
QALY: 0.44 vs 0.017 |
€34,534/QALY |
|||
|
Zhang et al. (2015)16 |
Advanced HCC |
SFN vs BSC |
Drugs, tests, and grade 3/4 AE |
$19,495 vs $897 |
QALY: 0.45 vs 0.27 |
$101,399/QALY |
|
Zhang et al. (2016)17 |
Advanced HCC |
SFN vs FOLFOX4 |
Drugs, administration, venous access management, nursing care, tests, hospitalization, AE, indirect costs |
$18,748 vs $6,876 |
QALY: 0.3935 vs 0.3808 |
$934,801/QALY |
|
Leung et al. (2016)18 |
Unresectable advanced HCC |
SFN vs SBRT |
drug costs, laboratory test, physician visits, pharmacy dispensing fee, administration and nursing care fee, AE |
NT$ 2,166,079 VS NT$1,197,039 |
QALY: 3.07 vs 2.81 |
NT$426,117 / QALY |
|
Rognoni et al. (2017)19 |
Intermediate HCC |
TARE vs SFN |
Drug costs, Exam, procedure, DRG |
€29,289 vs €31,149 |
QALY: 0.638 vs 1.178 |
€3,302/QALY (dominatd) |
|
Advanced HCC |
€30,750 vs €21,961 |
QALY: 0.568 vs 0.639 |
TARE dominant |
|||
|
Parikh et al. (2017)20 |
Advanced HCC |
SFN vs No therapy |
Diagnosis to the end of follow-up, drugs |
NA |
NA |
$224,914/LYG |
|
Zhao et al. (2017)21 |
Unresectable HCC |
SFN+TACE vs TACE |
Included inpatient visit, diagnostic and laboratory testing, medications, and procedures |
$44,542 vs $26,951 |
QALY:1.02 vs 0.71 |
$56,745/QALY |
|
Chen et al. (2018)9 |
Advanced HCC |
Full dose SFN vs TACE |
Direct medical costs in China |
$16,703 vs $10,642 |
QALY: 0.435 vs 0.375 |
$101,028.83/QALY |
|
Direct medical costs in USA |
$34,190 vs $95,061 |
QALY: 0.435 vs 0.375 |
SFN Dominant |
|||
|
Ho et al. (2018)22 |
Advanced HCC |
SFN vs SFN+Other |
Direct medical costs accrued from inpatient and outpatient care, and pharmacy visits |
NT$522,695 vs NT$957,483 |
QALY: 0.3837 vs 0.5432 |
NT$2,725,943/QALY |
|
Qin et al. (2018)23 |
Intermediate and Advanced HCC |
SFN vs FOLFOX4 |
Drugs, General ward, tests, AE treatment |
$12,798 vs $8,428 |
QALY: 0.42 vs 0.38 |
$128,559 /QALY |
HCC: Hepatocellular carcinoma, SFN: sorafenib, BSC: Best supportive care, LYG: life year gain, LT: liver transplantation, QALD: quality-adjusted life days, QALY: quality-adjusted life year, SBRT: Stereotactic body radiotherapy, TARE: transarterial radioembolization, TACE: transarterial chemoembolization, DRG: diagnosis related group, FOLFOX4: oxaliplatin plus infusional-fluorouracil/leucovorin
Table 4 Cost-effectiveness results of SFN in metastatic renal cell carcinoma
The cost-effectiveness analyses of sorafenib-containing therapy were conducted in five different countries including USA (n = 3), Italy (n = 3), Canada (n=1), Taiwan (n=2), and China (n=5). All of them except one study were developed in a single country. Markov model was applied for all of the studies in a 2 year to life-time time horizon. However, Parikh et al.20 did not report model, time horizon and perspective of study. 10 studies disclosed their source of funding.
The question of studies was focusing on the comparison of SFN, SBRT, FOLFOX4, TARE, TACE or Best Supportive Care (BSC) in the treatment of HCC in terms of cost-effectiveness. Three studies were sponsored by Sanofi Bayer. Four studies declined any financial support. Three studies did not announce, obviously. Three studies were supported by universities in China and Italy.
Sorafenib versus best supportive careMuszbec et al.12 developed a Markov cohort model to evaluate the cost-effectiveness of sorafenib versus BSC as first-line treatment in advanced HCC employing data from SHARP24 study. Costs were calculated in 2007 Canadian dollars with a time horizon of lifetime and a 5% discount rate from Canadian provincial Ministry of Health perspective in Canada. The resources used on medical costs were based on publicly available sources, and in the absences of published data experts’ opinion were used. The result illustrated that sorafenib was associated with the incremental effectiveness of 0.49 life years gained (LYG). As a result, the incremental cost effectiveness ratio (ICER) was estimated as Can$75,759 per LYG. Sensitivity analysis showed that the model is sensitive to overall survival (OS), time to progression (TTP) and BSC costs after progression. Finally, the authors who disclosed that supporting by Bayer HealthCare Pharmaceuticals, concluded that although sorafenib is more effective than BSC, it is not cost effective at the threshold of $50,000.
Vitale et al.13 compared sorafenib with no therapy before LT employing a Markov model. The model was run in a 10-year time horizon given a payer perspective in 2008. The result indicated an incremental quality adjusted life days (QALD) of 94 (1350 versus 1244 QALD) resulting in an ICER of €197/QALD. The calculated WTP of sorafenib in Italy was €346 per QALD. In conclusion, Sorafenib neoadjuvant therapy was found to be cost-effective in comparison to no therapy for patients waiting for LT.
In another study, Carr et al.14 adopted a Markov model to assess the cost-effectiveness of sorafenib versus BSC for advanced HCC from the perspective of third-party payer. OS and progression free survival (PFS) of sorafenib was derived from SHARP study. Costs were reported in 2007 dollar over a lifetime horizon. LYs was calculated as 1.58 for sorafenib and 1.05 for BSC, respectively. In addition, sorafenib was associated with an incremental cost of $32,835 compared to BSC ($40,639 versus $7,804) resulting in an ICER of $62,473/LYG. The economic evaluation showed that sorafenib was cost-effective compared BSC in USA. One-way sensitivity analysis found OS for sorafenib and BSC to be the most sensitive variables, followed by TTP with sorafenib, the cost of first-line treatment with sorafenib—no progression, and the cost of first-line treatment with BSC—no progression.
Cammà et al.15 compared cost-effectiveness of sorafenib full dose and sorafenib adjusted dose with BSC using a Markov model. Costs were calculated in 2012 euro with a time horizon of 5-year and a 3% discount rate from third-party managed-care payer perspective in Italy. The resources used on medical costs were based on the DRG tariffs and national ambulatory fees. The result illustrated that sorafenib dose adjusted in Barcelona Clinic Liver Cancer (BCLC) B and C stage was associated with the incremental effectiveness of 0.41 life years gained (LYG) and 0.28 QALYs. As a result, ICER was estimated as €25,874 per LYG and €34,534 per QALY for sorafenib dose-adjusted in BCLC B,C. Sensitivity analysis showed that the model is sensitive to an assumption on survival rates of BSC patients, sorafenib treatment duration, and type of survival distribution. Finally, the authors concluded that dose-adjusted sorafenib is more effective than BSC which was lower than the generally accepted societal WTP threshold.15
Similarly, Zhang et al.16 analyzed sorafenib and BSC in the treatment of advanced HCC. A Markov model was developed using 3% discount rate with the payer’s perspective. All costs were measured in US dollars. The result demonstrated an incremental QALY of 0.18 (0.45 versus 0.27 QALY) together with an incremental cost of $18,597.84 ($19,495.05 versus $897.21) resulting in an ICER of $101,399.11/QALY. The sensitivity analysis indicated that the model is sensitive to the cost of sorafenib, and duration of the PFS state for the sorafenib group. This study showed sorafenib is not a cost-effective strategy at a WTP of $20 301.00/QALY in China The study was not been sponsored.16
Parikh et al.20 investigated the cost-effectiveness of sorafenib in patients with advanced stage HCC using Medicare beneficiaries with HCC diagnoses from 2007-2009 in USA included 228 sorafenib-treated patients and 870 control patients. ICERs were calculated for sorafenib-treated and control patients. All costs were measured in US dollars in 2015. The results illustrated the median survival of the sorafenib-treated patients was 150.5 days versus 62 days for control patients. The median survival benefit was 31 days and sorafenib was not found to be cost-effective with an ICER of $224,914 per life year gained.
Sorafenib versus SBRTLeung et al.18 stated sorafenib was effective in comparison with SBRT in patients with advanced HCC; however, due to the higher costs, it was not found to be cost-effective. Costs were calculated in 2015 new Taiwan dollars with 5-year time horizon and a 3% discount rate with National Health Insurance perspective. The results indicated that SFN was associated with the incremental effectiveness of 0.26 QALYs. The ICER was estimated $114,795 per QALY for sorafenib versus SBRT. Sensitivity analysis demonstrated that the model is sensitive to the utility of progressive disease for the sorafenib treatment, utility of PFS for SBRT, utility of PFS for sorafenib, utility of PFS to progression disease for SBRT and transition probability of progressive disease to death for SBRT. Leung et al.18 stated that the study had not any sponsor.
Sorafenib versus FOLFOX4Zhang et al.17 compared the cost and effectiveness of sorafenib versus FOLFOX4 in 2016 from a Chinese societal perspective in the first-line treatments for advanced hepatocellular carcinoma. A Markov cohort model was used with a 10-year time horizon. ICERs of FOLFOX4 versus sorafenib was calculated to be $934,801.57/QALY. The sensitivity analysis showed that the model is sensitive to duration of PFS state for sorafenib group, cost of PFS state for sorafenib group, cost of sorafenib and utility of PD state. It was resulted that FOLFOX4 was cost-effective compared to SFN. The study demonstrated sorafenib is not a cost-effective strategy at a WTP of $20,301.00/QALY in China.
In another study, Qin et al.23 compared FOLFOX4 versus sorafenib in 2017 from healthcare system and patient’s perspective for advanced HCC in China. A Markov model was developed using 5% discount rate over a lifetime time horizon. The results demonstrated that FOLFOX4 dominated sorafenib with lower costs (FOLFOX4: US$ 8428 sorafenib: US$ 12,798) and higher QALYs (FOLFOX4: 0.42; sorafenib: 0.38) per patient. Sorafenib was not found to be a cost-effective strategy at a WTP of $22,073/QALY in China. According to one-way sensitivity analyses, the result was most sensitive to FOLFOX4 and sorafenib survival (PFS and OS) and the cost of sorafenib therapy.
Sorafenib versus TARERognoni et al.19 estimated the cost-effectiveness of TARE versus sorafenib in intermediate and advanced HCC using LY and QALY. Costs were calculated in 2016 euro with a lifetime horizon and a 3.5% discount rate from healthcare system perspective. In intermediate stage, the result indicated an incremental QALY of 0.540 (1.178 versus 0.638 QALY) together with an incremental cost of €1,782 (€31,071 versus €29,289) resulting in an ICER of €3,300/QALY. In advanced stage, the results showed that sorafenib was dominated compared to TARE due to its higher cost and lower efficacy in Italy. This study which was funded by ASBM Srl through an unrestricted grant to CERGAS, Bocconi University (Milan, Italy).
Sorafenib versus TACEChen et al.9 developed a Markov model to judge the cost-effectiveness of sorafenib versus TACE for advanced HCC. Costs were calculated in 2016 dollars with a 2-year time horizon and a 3% discount rate from healthcare system perspective. The most important parameter that has affected the results was the mortalities of compensated cirrhotic patients without and with progression taking dose-adjusted sorafenib, in both countries. In this study, full dose and adjusted dose were compared with TACE. Full and dose-adjusted earned 0.435 and 0.482 QALY, respectively while TACE gained 0.375 QALY. The ICER of full-dose sorafenib versus TACE was $101,028.83/QALY in China whereas full-dose sorafenib was a dominant strategy (ICER of -$1,014,507.20/ QALY) compared with TACE in the USA. Compared to full-dose sorafenib, dose-adjusted sorafenib was the dominant strategy with the negative ICERs in both China (−$132,238.94/QALY) and the USA (−$230,058.09/QALY). Chen et al. disclosed that the study sponsored by National Natural Science Foundation of China and Pearl River S&T Nova Program of Guangzhou, China.
Sorafenib + TACE versus TACEZhao et al.21 analyzed TACE in comparison to TACE plus sorafenib in unresectable hepatocellular carcinoma. A Markov cohort model was adopted using 3% discount rate from a healthcare system perspective in China. All the costs were measured in 2015 US dollars with a lifetime time horizon. The result demonstrated an incremental QALY of 0.31 (1.02 versus 0.71 QALY) together with an incremental cost of $17,591 ($44,542 versus $26,951) resulting in an ICER of $56,745/QALY. In conclusion, TACE was found to be cost-effective strategy in comparison with TACE plus sorafenib. The sensitivity analyses showed the cost post TACE-sorafenib therapy with stable state was most influence factor on ICER. This study has not been sponsored.
Sorafenib versus sorafenib + other therapyHo et al.22 evaluated the cost-effectiveness of sorafenib versus sorafenib plus other therapy in patients with advanced HCC. A Markov model was developed using 3% discount rate with 5-year time horizon. All costs were calculated in 2014 new Taiwan dollar with healthcare system perspective. Analysis showed that combination treatment with sorafenib was associated with an incremental cost of NT$434,788 and produced 0.1595 QALY resulting in an ICER of NT$2,725,943/QALY. Thus, SFN plus other therapy was not cost-effective treatment in Taiwan at a WTP of NT$ 2,213,145/QALY. The sensitivity analyses demonstrated that the model was sensitive to health utility values and monthly costs accrued in the progression- free survival state of the combination therapy group. this study funded by China Medical University grant.
Quality assessment (QHES)The results of the quality assessment using the QHES instrument have been presented in Table 5. It was found that the quality of the included studies was at a high level (Mean QHES Score: 92.4). The objectives of studies were clearly presented in all studies (Question 1). The perspective of the analysis had been stated in all studies. However, just one study had state justification for the reasons of selection (Question 2). The best available source of data was utilized by all the studies (Question 3). When subgroup analyses were conducted, the groups were pre-specified (Question 4) and all studies handle uncertainty in an appropriate way but three studies including Zhang et al., Zhao et al. and Parikh et al.20 conduct only a one-way sensitivity analysis (Question 5). All studies performed incremental analysis between alternatives for effectiveness and costs (Question 6). In addition, all of the studies provided detailed information on the methods used to derive effectiveness (Question 7 & Question 8). Moreover, all studies measured costs appropriately (Question 9).
|
Study |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Muszbek et al.12 |
√ |
± |
± |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
94 |
|
Vitale et al.13 |
√ |
± |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
± |
√ |
√ |
√ |
√ |
√ |
X |
92 |
|
Carr et al.14 |
√ |
± |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
98 |
|
Cammà et al.15 |
√ |
± |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
X |
95 |
|
Zhang et al.16 |
√ |
± |
√ |
√ |
± |
√ |
√ |
± |
√ |
√ |
√ |
± |
√ |
√ |
√ |
√ |
86 |
|
Zhang et al.17 |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
100 |
|
Leung et al.18 |
√ |
± |
√ |
√ |
√ |
√ |
√ |
± |
√ |
√ |
√ |
± |
√ |
√ |
√ |
√ |
90.5 |
|
Rognoni et al.19 |
√ |
± |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
98 |
|
Parikh et al.20 |
√ |
± |
√ |
√ |
± |
√ |
√ |
√ |
√ |
√ |
√ |
± |
√ |
√ |
√ |
√ |
89.5 |
|
Zhao et al.21 |
√ |
√ |
± |
√ |
± |
√ |
± |
± |
√ |
± |
√ |
X |
± |
√ |
√ |
X |
68 |
|
Chen et al.9 |
√ |
± |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
98 |
|
Ho et al.22 |
√ |
± |
√ |
√ |
√ |
√ |
√ |
± |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
94.5 |
|
Qin et al.23 |
√ |
± |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
98 |
Table 5 ChemicalQuality assessment of studies using the QHES instrument
The primary outcome measures had been clearly stated in all studies and other relevant outcomes were addressed except Vitale et al. study (Question 10). In addition, the reliability and validation of health outcome measures had been tested before (Question 11). The model had been clearly explicated by the authors of all studies (Question 12). Then, the justification for the choice of the model and discussion on results, assumptions, and limitations had been given by all the studies (Question 13). The direction and magnitude of potential bias had also been discussed by the authors of all studies (Question 14), and the conclusions drawn by the authors of all studies were based on the study results and sounded reasonable (Question 15). Finally, Vitale et al., Camma et al. and Parikh et al. do not disclosure the source of funding for the study (Question 16).
Discussion
This study is the first systematic review of the economic evaluation studies of sorafenib in HCC treatment. The cost-effectiveness of sorafenib has been evaluated in several stage of disease against various treatment regimens. The findings in this study point to an incremental cost per QALY in the range of $-1,014,507 to $934,801, depending on whether the disease stage is advanced or not and which comparator is utilized.
Overall, the results indicated that Sorafenib could considered as a cost-effective treatment option for patients with HCC. Briefly, compared to BSC, sorafenib is associated with higher cost and higher efficacy. However, the resulting ICERs were higher than WTP thresholds. Nevertheless, the best result was achieved when BSC compared to adjusted dose of sorafenib15. The highest ICER resulted when comparing sorafenib to FOLFOX17. TARE was selected as dominant strategy 19, while TACE was dominated in comparison to sorafenib in USA.9
Most of studies mentioned that the model was sensitive to clinical effectiveness (OS or PFS) and drug acquisition cost. In addition, some of them also showed sensitivity to health state utility17,18,22. Not only being sensitive to clinical effectiveness somewhat makes the result questionable, but also extrapolating clinical efficacy and treatment persistency from short-term RCTs to a long treatment period carries significant uncertainty and requires additional assumptions that are not well acknowledged in the literature.
For transferability of the results of these studies, in addition to noting different health care settings, different costs, different medical procedures, etc., willingness to pay should also be consider an important contributing factor. It should be also noted that willingness to pay threshold is not a rigid cutoff all over the world. For example, in the United States, a threshold of $50,000 per QALY is generally accepted to assess the cost-effectiveness of an intervention. Furthermore, health care authorities in China and Taiwan consider a treatment as a cost-effective strategy if it results in an ICER of ≤$20,301/QALY and ≤$NT2,213,145/QALY, respectively. In the Italy advocated a cut-off at an ICER of £38,000/QALY. So, the results should be interpreted with a view to the specific willingness to pay threshold in different jurisdictions.
Generalizability of the trials to the wider patient population is another limitation in targeted studies. Most of the patients included in the clinical trials have had good performance and prognosis. Accordingly, the generalizability of estimated overall survival to the wider patient population can prove a limiting factor. In addition, the generalizability of cost-effectiveness studies, which have been conducted in a specific country, to other health care settings may be limited due to the differences in costs between different countries such as developed and developing countries.
Conclusion
In sum, this review revealed that sorafenib was more effective with higher cost than its comparators except TARE and could be considered as a cost-effective treatment option for patients with metastatic HCC. The results should be interpreted with a view to the specific health care settings and willingness to pay threshold in different jurisdictions.
Funding
No funding has been received for this research.
Conflict of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Llovet JM, Lencioni R, Di Bisceglie AM, et al. EASL-EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. Eur J Cancer. 2012;56(4):908-943. doi:10.1016/j.jhep.2011.12.001
- Thein H-H, Isaranuwatchai W, Campitelli MA, et al. Health care costs associated with hepatocellular carcinoma: A population-based study. Hepatology. 2013;58(4):1375-1384. doi:10.1002/hep.26231
- Fitzmaurice C, Dicker D, Pain A, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505. doi:10.1001/jamaoncol.2015.0735
- Tang A, Hallouch O, Chernyak V, Kamaya A, Sirlin CB. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdominal Radiology. 2017:1-13.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358-380. doi:10.1002/hep.29086
- Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. doi:10.1016/j.jhep.2018.03.019
- Omata M, Cheng A-L, Kokudo N, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317-370. doi:10.1007/s12072-017-9799-9
- Zhang T, Ding X, Wei D, et al. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs. 2010;21(3):326-332. doi:10.1097/CAD.0b013e3283350e26
- Chen S, Peng Z, Wei M, et al. Sorafenib versus Transarterial chemoembolization for advanced-stage hepatocellular carcinoma: a cost-effectiveness analysis. BMC Cancer. 2018;18(1):392.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg Lond Engl. 2010;8(5):336-341. doi:10.1016/j.ijsu.2010.02.007
- Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, Von Der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2014;50(1):40-49. doi:10.1016/j.ejca.2013.08.008
- Muszbek N, Shah S, Carroll S, et al. Economic evaluation of sorafenib in the treatment of hepatocellular carcinoma in Canada. Curr Med Res Opin. 2008;24(12):3559–3569.
- Vitale A, Volk ML, Pastorelli D, et al. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: A cost-benefit analysis while awaiting data on sorafenib safety. Hepatology. 2010;51(1):165-173. doi:10.1002/hep.23260
- Carr BI, Carroll S, Muszbek N, Gondek K. Economic evaluation of sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25(11):1739-1746. doi:10.1111/j.1440-1746.2010.06404.x
- Cammà C, Cabibbo G, Petta S, et al. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57(3):1046-1054. doi:10.1002/hep.26221
- Zhang P, Yang Y, Wen F, et al. Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015;27(7):853-859. doi:10.1097/MEG.0000000000000373
- Zhang P, Wen F, Li Q. FOLFOX4 or sorafenib as the first-line treatments for advanced hepatocellular carcinoma: A cost-effectiveness analysis. Dig Liver Dis. 2016;48(12):1492-1497. doi:10.1016/j.dld.2016.07.007
- Leung HWC, Liu C-F, Chan ALF. Cost-effectiveness of sorafenib versus SBRT for unresectable advanced hepatocellular carcinoma. Radiat Oncol. 2016;11(1):69. doi:10.1186/s13014-016-0644-4
- Rognoni C, Ciani O, Sommariva S, Tarricone R. Real-World Data for the Evaluation of Transarterial Radioembolization versus Sorafenib in Hepatocellular Carcinoma: A Cost-Effectiveness Analysis. Value Health. 2017;20(3):336-344. doi:10.1016/j.jval.2016.09.2397
- Parikh ND, Marshall VD, Singal AG, et al. Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: An analysis of the SEER–Medicare database. Hepatology. 2017;65(1):122–133.
- Zhao R-C, Zhou J, Wei Y-G, et al. Cost-effectiveness analysis of transcatheter arterial chemoembolization with or without sorafenib for the treatment of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2017;16(5):493-498. doi:10.1016/S1499-3872(17)60009-2
- Ho J-CC, Hsieh M-LL, Chuang P-HH, Hsieh VC-RR. Cost-Effectiveness of Sorafenib Monotherapy and Selected Combination Therapy with Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Value Health Reg Issues. 2018;15:120-126. doi:10.1016/j.vhri.2017.12.012
- Qin S, Kruger E, Tan SC, Cheng S, Wang N, Liang J. Cost-effectiveness analysis of FOLFOX4 and sorafenib for the treatment of advanced hepatocellular carcinoma in China. Cost Eff Resour Alloc. 2018;16(1):29.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390.