Volume : 0 | Issue : 0
Research
Analysis of the role of acidity and tea substrate on the inhibition of α-amylase by Kombucha
Mallory Dickmann,2 Rebecca Schneider,2 Samantha Armando2, Katherine Seehusen,2 Patrick Hager,2 Michael J Strauss,2 Francis M Mann1
1Department of Chemistry, University of Wisconsin-Parkside, USA
2Department of Chemistry, Winona State University, USA
Received: October 17, 2017 | Published: November 07, 2017
Abstract
Kombucha tea (KT) is an acidic, fermented, tea-based beverage associated with a variety of reported health benefits. Specifically, KT consumption has previously elicited hypoglycemic responses in alloxan-induced diabetic rats, implying KT consumption may alleviate symptoms of diabetes. One method by which KT may act as a hypoglycemic agent involves inhibition of digestive glucosidases, such as α-amylase. Indeed, KT demonstrated an IC50 of 0.16±0.06% (mL/U) during in vitro pancreatic amylase assay, while unfermented black tea failed to inhibit the enzyme. Inhibition of α-amylase by KT may be due to pH-dependent inactivation of the enzyme, biotransformation of tea catechins, or a production of an inhibitory factor during fermentation. Production of tea-free kombucha (MT) allowed further analysis of the roles of tea catechins on the inhibitory capacity of the beverage. MT demonstrated higher inhibitory dose than KT concentration at acidic pH (IC50 = 6.3±5.4% (mL/U)), but MT inhibitory activity was completely abrogated by pH adjustment. Conversely, inhibitory capacity of pH adjusted KT was reduced less than 10-fold (IC50 = 1.2±0.23% (mL/U), however, complete inhibition was not achieved. Together these results support a role for both pH and biotransformation of tea compounds in the inhibitory mechanism of Kombucha tea on the enzyme α-amylase, however, de novo fermentative production of an α-amylase inhibitor is unlikely.
Introduction
Kombucha tea (KT) is a fermented tea beverage consumption of which is linked with numerous health benefits including antioxidant, anti-inflammatory, anti-cancer, hypoglycemic, and antimicrobial effects.1 Previous treatment of alloxan-induced diabetic rats with KT resulted in a greater hypoglycemic response than black tea alone, supporting the role of KT or KT-derived metabolites in the treatment and control of blood sugar disorders. The mechanism was unclear, though it was noted that the rats exhibited decreased pancreatic α-amylase activity upon treatment with KT,2 warranting further analysis of the inhibitory capacity of KT on the digestive enzyme.
KT is typically composed of brewed tea, sugar, and a symbiotic culture of bacteria and yeast (SCOBY), which forms a carbonated, acidic beverage during a 7-21 day fermentation period.1 While acetic acid is the primary organic acid produced, lactic, D-glucoronic, and citric acid have all been observed with culture dependent variation.3-4 Tea-derived catechins and phenolic molecules are also found in the finalized product in varying concentrations.5
Tea catechins have many demonstrated bioactivities including antioxidant, anti-inflammatory, and hypoglycemic properties.6 Previously, tea catechins were proposed as hypoglycemic agents due to their ability to inhibit enzymes in carbohydrate metabolism, namely α-amylases and glucosidases.7 However, limitations in the bioavailability and inhibitory capacity of catechins limit their applicability as lead molecules. In situ investigations indicate that the efficacy of these compounds as amylase inhibitors is directed related to oxidation at specific sites on the ring structure.8 This indicates oxidative biotransformation may increase inhibitory capacity of the tea phenolics, providing appropriate lead molecules for pharmaceutical agents.
While the mechanism by which KT induces hypoglycemia has not been characterized in vivo, it has been proposed that substrate tea catechins are responsible for the majority of the inhibitory capacity of the beverage.5-9 However, as KT is a complex concoction of known and unknown compounds, alternative explanations, such as pH-dependent enzyme inactivation, culture-synthesized compounds, or biotransformation of the tea substrate may be responsible. This study aims to explore the mechanism by which KT inhibits the digestive enzyme α-amylase in vitro by exploring the roles of the pre-fermentative substrate, pH, and fermentative products on the activity of α-amylase.
Materials and methods
Kombucha SCOBY was purchased from Quill Grp. and fulfilled by Amazon.com. Tea was brewed using Lipton® black tea (Unilever, Englewood Cliffs, NJ, USA). All other reagents and enzymes were purchased from Sigma Aldrich (St. Louis, MO, USA).
Preparation of kombucha black tea: One tea bag was brewed for 10 minutes in 315 mL of 90°C water containing 8% (g/mL) dissolved sucrose. The tea bag was removed and the liquid was cooled on ice to 37°C. A volume of 26.5 mL 5% acetic acid solution was added to the liquid prior to inoculation with the SCOBY. SCOBY inoculation occurred by aseptic transfer of the SCOBY mat from into the tea substrate. The SCOBY fermented for 14 days at 28°C which allowed cell division and production of progeny in the form of a mixed microbial mat found on top of the liquid. The progeny was then removed to initiate fermentation on new tea substrate (termed one ‘subculture’). Prior to inoculation of fresh media, the culture mat was aseptically trimmed to ensure equal mass transfer into each subculture. Three sequential subcultures were performed prior to collection of any Kombucha liquid for analysis, with the SCOBY progeny formed during the third culturing used to inoculate fresh tea substrate for the first set of liquid samples. SCOBY showing signs of contamination by mold of insects were discarded. Liquid samples were collected from four subsequent subcultures (e.g. subculture generations 4-7 from the original SCOBY) for analysis after 14 days. Samples of untreated brewed tea were prepared by replacing acetic acid volume with water and freezing to prevent spontaneous fermentation.
Preparation of tea-free kombucha: Kombucha SCOBY was fermented in liquid approximating the nutritional content of brewed Lipton tea as described by the United States Department of Agriculture Nutrient Database for Standard Reference for tea brewed with tap water.10 Briefly, 281.3 mL of water containing 8% sucrose was warmed to 90°C. Trace nutrients of magnesium chloride heptahydrate (0.02%), potassium chloride (0.06%), sodium chloride (0.005%), and caffeine (0.018%) were directly dissolved. The liquid was cooled to 37°C and 33.75 mL of a 10x Mock Tea stock (5.4 mg Riboflavin, 35.8 mg ZnSO47H2O, 1.5 g KH2PO4, and 41.25 mg FeSO4·7H2O per liter) was added. Kombucha SCOBY was used to inoculate the media by aseptic transfer of SCOBY mat into tea-free substrate. The SCOBY fermented for 14 days at 28°C which allowed cell division and production of progeny in the form of a mixed microbial mat found on top of the liquid. The progeny was then removed to initiate fermentation on new tea substrate (termed one ‘subculture’). Prior to inoculation of fresh tea-free media, the SCOBY mat was aseptically trimmed by sterile scalpel to ensure equal mass transfer of SCOBY into subcultures. As previously described, three sequential subcultures were performed prior to collection of any Kombucha liquid for analysis. SCOBY showing signs of contamination by mold of insects were discarded. Liquid samples were collected from four subsequent subcultures (e.g. subculture generations 4-7) for analysis after 14 days of fermentation.
Kombucha fermentation time course: Three samples of subcultured seventh-generation Kombucha fermented on black tea substrate were fermented for extended time periods. Kombucha fermentation of these was performed for a total of 56 total days. A volume of 25 mL was aseptically removed from each culture at 14, 42, and 56 days. Samples were frozen at -20°C until the completion of the time course, after which analysis were performed.
Amylase assay: Kombucha tea(s), tea, and tea-free substrate were pH adjusted and degassed under vacuum for one hour prior to analysis. Due to the sugar content of Kombucha, negative control assays were required, which were composed of Kombucha dilutions incubated with amylase in the absence of starch. Dilutions were prepared in dIH2O in concentrations ranging from 25% to 0.1% (v/v). Assays were incubated with 0.44 mg/mL soluble starch in 20 mM phosphate buffer, pH 6.9. Porcine pancreatic amylase was diluted to 1 U/mL in cold dIH2O and used to initiate assays. Assays proceeded for five minutes at 25°C and were stopped by addition of 1 mL coloring reagent (38.5 mM 3,5-dinitrosalicylic acid, 0.425 M Sodium potassium tartrate tetrahydrate, 0.2 M Sodium hydroxide in dIH2O) and incubation at 100°C for five minutes. Reactions were quenched by pH-adjustment with 30 mM Sodium acetate buffer, pH 3.5. The assays were diluted ¼ in dIH2O and analyzed at 540 nm. Duplicate assays were performed, and data presented represents the average duplicate assays of at least three trials of unique Kombucha tea, or tea-free substrate preparations. Data was analyzed graphically using Kaleidagraph (Synergy Software, Eden Prairie, MN, USA). Dose response curves were fit to a standard four-parameter dose-response logistic model, as is appropriate for determining IC50 values for data with variable asymptotes.11 The equation for the curve fit is as follows: a+(b-a)/(1+(x/c)^d) where a = y-min, b = y-max, c = IC50, d = slope. Curve fits were applied with tolerance of 1% deviation.
Results
Effect of kombucha fermentation on the inhibition of α-amylase by black tea
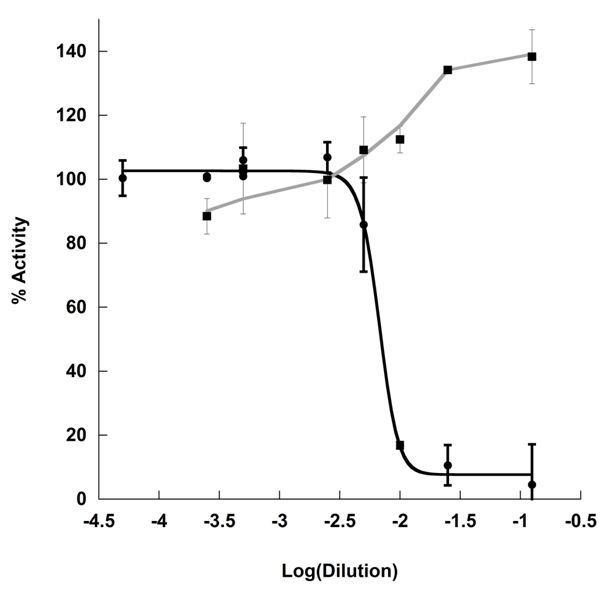
Kombucha tea is a fermented tea beverage. Phenolic compounds found in tea are known inhibitors of starch hydrolases,12 and thus it is possible that the tea substrate provides the source of inhibition. However, previous studies indicate that Kombucha fermented tea increases the inhibitory activity of the tea in vivo.2 To further probe the relationship between the tea substrate and starch hydrolase inhibition, KT was fermented on black tea substrate in the presence of 8% sucrose for 14 days and compared to black tea brewed to the same concentration (BT). The effect of KT and BT on α-amylase activity was assayed using porcine pancreatic α-amylase in the presence of 0.44 mg/mL soluble starch with detection by 3,5-dinitrosalicylic acid (Figure 1). Inhibitory concentrations were described in terms of mL of analyte per unit of enzyme (mL/U). As assayed, BT failed to produce any inhibitory effect. KT resulted in an IC50 of 0.16±0.06% (mL/U) (Figure 1). The absence of inhibitory activity by the tea substrate is likely to be due to low extraction of the catechins into aqueous conditions during the brewing process,13 as tea phenolics are confirmed inhibitors of α-amylase12 and yet no inhibition was observed.
Examination of neutralization on the inhibitory capacity of kombucha tea on α-amylase
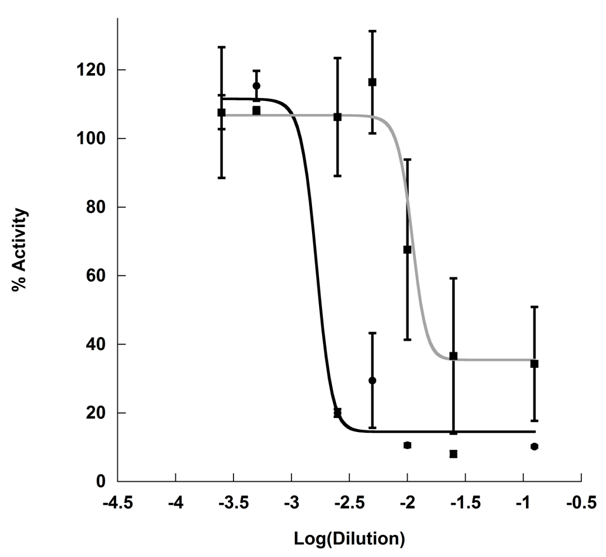
Initially, the inhibitory effect of KT on α-amylase activity was believed to be due to the pH discrepancy between KT (pH 2.68±0.19) and BT (pH 5.01±0.05). Fermentation with Kombucha SCOBY results in the production of a variety of organic acids in differing concentrations, namely: acetic, lactic, and glucuronic acid with some reports indicating the presence of additional acids, such as ascorbate, citric, gluconic, malic, oxalic, quinic acid the beverage.4,5,14 The identity of organic acids varies with the host SCOBY, though the terminal pH of the Kombucha tea appears to remain in the range of 2.5-3.0.1,4 As such, exogenous addition of organic acids to BT substrate was not practical as the identity of the exact mix of organic acids present in the KT was unknown. Furthermore, pH adjustment with strong acid was also impractical as typical anions have α-amylase activating activity.15 Instead, KT pH was neutralized to match that of BT, and the assay was repeated. KT exhibited increased inhibitory capacity over BT even the same pH. Notably, pH adjustment of KT resulted 10-fold reduction in inhibitory dose and incomplete inhibition (IC50=1.2±0.23% (mL/U), Activity minimum = 49.8±5.1%) (Figure 2).
Effect of fermentation duration of Kombucha tea on inhibitory capacity of α-amylase
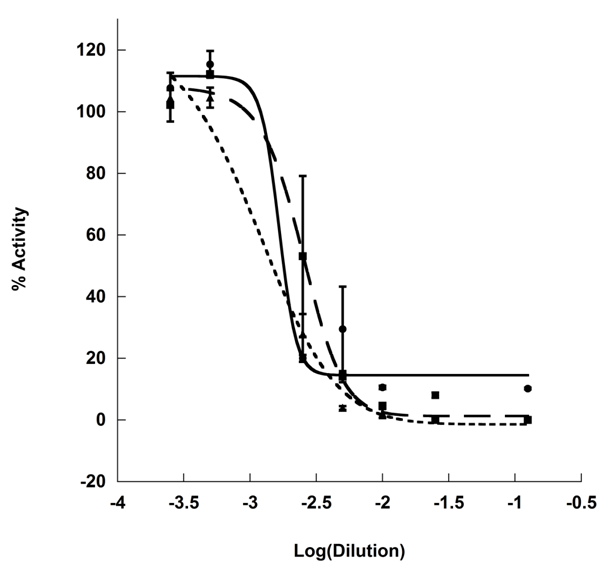
Further analysis of the inhibitory effect of KT indicated that inhibition was due to a fermentative product of the SCOBY, as increased fermentation periods resulted in greater inhibitory capacity and lower IC50 values (Table 1) over increasing 2-week fermentation periods (Figure 3). Analysis of post-fermentative acidity fails to correlate to inhibition to pH as pH was initially measured as 2.54±0.14 at 14 days post-innoculation and actually rose during the subsequent 28 day period to pH=3.03 while IC50 was reduced by half (0.16±0.06 mL/U to 0.08±0.034 mL/U, respectively). During the final fermentation period, the pH once again fell to 2.52 and IC50 was reduced 8-fold. The variability of pH over the fermentation period is unsurprising as various groups have reported the variation of organic acid content during Kombucha fermentation,4,5 and all pH measurements fall within the typical range of reported Kombucha fermentations.
| pH | IC50 (mL/U α-amylase) ± Std. Deviation | Minimum Activity (% control ± % Deviation) | |
|---|---|---|---|
| Mock Tea (2 wk) | 2.34±0.11 | 6.3±0.054 | -- |
| Black Tea (2 wk) | 2.54±0.14 | 0.16±0.06 | 14.52±5.4 |
| Black Tea (6 wk) | 3.03 | 0.08±0.034 | 1.290±3.1 |
| Black Tea (8 wk) | 2.52 | 0.01±0.59 | 0.000±5.1 |
| pH-Adjusted (2 wk) | Minimum Activity (% control ± %) | ||
| Mock Tea, pH 5.0 | 5.05±0.15 | -- | -- |
| Black Tea, pH 5.0 | 5.01±0.13 | 1.20±0.23 | 49.8±5.1 |
Table 1 pH and IC50 values for black tea (BT) and tea-free (MT) substrates in the presence and absence of Kombucha fermentation.
pH and IC50 of black tea substrate and KT at 2,4, and 6 weeks post-innoculation were compared to pre- and post-fermentative MT pH and IC50. Data represents the average ± standard deviation of triplicate analysis of three unique samples where error is provided. Data from 6- and 8-week black tea samples represent triplicate analysis of single samples.
Previously it has been demonstrated that KT fermentation results in the reduction of concentration of some tea catechins.5 Jayabalan et. al. hypothesized that the SCOBY secretes enzymes that cleaved the flavonoid ring structure or phenolic conjugation points, resulting in structural rearrangement.5 Common rearrangement involve depolymerization of theaflavins and thearubigins and cleavage of gallic acid from galloylated structures.16 Both such rearrangements can result in increased availability of phenolic compounds associated with α-amylase inhibition (e.g. gallic acid, epigallocatechin, and epigallocatechin gallate, specifically).7 Alternately, SCOBY-dependent de novo biosynthesis of an inhibitory compound was also possible. To probe the likelihood of these two possibilities, a tea-free substrate was developed to allow growth of the SCOBY in the absence of tea catechins and probe the role of tea phenolics in the production of inhibitory compounds during Kombucha fermentation.
Examination of the role of tea phenolics on production of α-amylase inhibitors by Kombucha fermentation
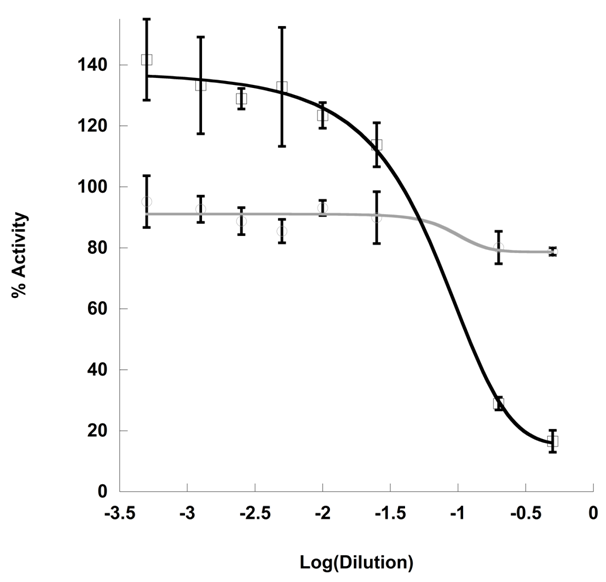
Tea-free Kombucha (MT) fermented for 14 days on 8% sucrose in the presence of vitamins, minerals, and caffeine concentrations analogous to concentrations found in BT resulted in similar pH to that of KT (2.54±0.14 for MT and 2.34±0.11 for KT) (Table 1). Dose dependent α-amylase inhibition was measured in the presence of MT and KT. MT resulted in reduced inhibitory dose (IC50=6.3±0.054 (mL/U)), however, minimum activity levels were comparable to KT (Figure 4). Unlike KT, MT-dependent inhibition was determined to be directly related to acidity, as pH adjustment of the MT to 5.05±0.15 completely alleviated inhibition (Figure 4).
Discussion and conclusion
Previously, the mechanism of action of Kombucha-induced starch hydrolase inhibition was theorized to be due to presence of tea catechins, as many phenolic compounds are known to inhibit starch hydrolases,7 though fermentation with Kombucha SCOBY resulted in greater inhibitory properties in vivo.2 In agreement with previous studies, KT demonstrated the expected inhibitory effect on α-amylase in these experiments. However, black tea failed to inhibit the enzyme, and appeared to increase α-amylase activity at high concentrations. As black tea catechins have demonstrable inhibitory action on α-amylase,7,12 this was initially surprising, however, further analysis of the literature indicates that the source and brewing conditions of the black tea may have resulted in very low concentrations of the bioactive molecules,17 placing the concentration of bioactive catechins too low for detection in our assay, and potentially stabilizing the enzyme with extracted ions, such as the chlorine ion.15 Though black tea did not inhibit α-amylase, Kombucha fermentation of black tea demonstrated α-amylase inhibition in a dose-dependent manner. This effect appears to increase with duration of fermentation period, but is not directly related to pH.
Post-fermentation pH analysis demonstrated that the final pH of KT was well outside of the maximal activity of α-amylase,18 and the KT was re-assayed after partial neutralization to the pH of brewed black tea. Though this resulted in a greater minimum activity, dose-dependent inhibition was still observed in the partially neutralized KT, indicating that fermentation of the black tea substrate resulted in production of a compound or compounds with greater inhibitory capacity then black tea alone. It was unclear whether the inhibitory molecules were produced independently of the tea phenolics or whether the Kombucha SCOBY transformed compounds in the tea substrate into compounds with greater inhibitory capacity.
In order to further probe the role of tea in the inhibitory activity of Kombucha, fermentation was performed in the absence of tea catechins. Two week fermentation on tea-free substrate (MT) resulted in 40-times greater IC50 but analogous minimum activity to KT despite similar pH. Furthermore, unlike KT, the inhibitory capacity of MT was completely abrogated by pH-adjustment (Figure 4) indicating that pH was largely responsible for α-amylase inhibition in the absence of tea phenolics.
Taken together, these results support the conclusion that the inhibitory effect of black tea derived Kombucha is due to a combinatorial effect of organic acids produced during fermentation and fermentation-dependent activation of tea derived compounds. Kombucha fermentation appears to activate tea catechins, or other tea derived compounds, to act as greater inhibitors of starch hydrolases such as α-amylase. Further studies attempting to isolate and characterize the inhibitory components of the Kombucha tea are required to completely characterize the mechanism of inhibition.
Conflict of interest
The authors wish to declare no conflicts of interest.
Acknowledgement
The authors wish to acknowledge Winona State University and University of Wisconsin-Parkside for funding, facilities, and instrumentation required to complete and publish this research.
References
- Dufresne C, Farnworth E. Tea, Kombucha, and health: a review. Food Research International. 2000;33(6):409−421.
- Aloulou A, Hamden K, Elloumi D, et al. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Altern Med. 2012;16:12−63.
- Jayabalan R, Malbaša RV, Lončar ES, et al. A Review on Kombucha Tea—Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Comprehensive Reviews in Food Science and Food Safety. 2014;13(4): 538−550.
- Neffe-Skocińska K, Sionek B, Ścibisz I, et al. Acid contents and the effect of fermentation condition of Kombucha tea beverages on physicochemical, microbiological and sensory properties. CyTA - Journal of Food. 2017;15(4):601−607.
- Jayabalan R, Marimuthu S, Swaminathan K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chemistry. 2007:102(1):392−398.
- Wierzejska R. Tea and health--a review of the current state of knowledge. Przegl Epidemiol. 2014;68(3):501−506,595−599.
- Xiao J, Ni X, Kai G, et al. A review on structure-activity relationship of dietary polyphenols inhibiting alpha-amylase. Crit Rev Food Sci Nutr. 2013;53(5):497−506.
- Lo Piparo E, Scheib H, Frei N, et al. Flavonoids for controlling starch digestion: structural requirements for inhibiting human alpha-amylase. J Med Chem. 2008;51(12):3555−3561.
- Watawana MI, Jayawardena N, Choo C, et al. Application of the Kombucha ‘tea fungus’ for the enhancement of antioxidant and starch hydrolase inhibitory properties of ten herbal teas. Food Chem. 2016;194:304−311.
- US Department of Agriculture, ARS, Nutrient Data Laboratory, USDA National Nutrient Database for Standard Reference. 2015, Release 28.
- Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharm Stat. 2011;10(2):128−134.
- Hara Y, Honda M. The Inhibition of α-Amylase by Tea Polyphenols. Agricultural and Biological Chemistry. 1990;54(8): 1939−1945.
- Astill C, Birch MR, Dacombe C, et al. Factors Affecting the Caffeine and Polyphenol Contents of Black and Green Tea Infusions. J Agric Food Chem. 2001;49(11):5340−5347.
- Malbaša RV, Lončar ES, Vitas JS, et al. Influence of starter cultures on the antioxidant activity of kombucha beverage. Food Chemistry. 2011;127(4):1727−1731.
- Aghajari N, Feller G, Gerday C, et al. Structural basis of α-amylase activation by chloride. Protein Sci. 2002;11(6):1435−1441.
- Chen ZY, Zhu QY, Tsang D, et al. Degradation of Green Tea Catechins in Tea Drinks. J Agric Food Chem. 2001;49(1):477−482.
- Lakenbrink C, Lapczynski S, Maiwald B, et al. Flavonoids and Other Polyphenols in Consumer Brews of Tea and Other Caffeinated Beverages. Journal of agricultural and food chemistry. 2000;48(7):2848−2852.
- Ishikawa K, Matsui I, Honda K, et al. The pH dependence of the action pattern in porcine pancreatic α-amylase-catalyzed reaction for maltooligosaccharide substrates. Arch Biochem Biophys. 1991;289(1):124−129.