Volume : 1 | Issue : 1
Research
Formation of volatile compounds in double roasted antakya coffee
Müge ARKADAŞ, Yahya Kemal AVŞAR
Department of Food Engineering, Mustafa Kemal University,Turkey
Received: February 06, 2018 | Published: March 07, 2018
Abstract
In this study, volatile compounds of Antakya coffee were determined by using high vacuum distillation and gas chromatography-mass spectrometry techniques (GC-MS). Green coffee beans were roasted up to 235°C and the samples were taken in 10 minute intervals. GC-MS results revealed the present of 36 volatiles (5 pyrroles, 9 pyrazines, 10 furans and 9 miscallenous compounds). Formation of thermally produced volatile compounds began at 140°C. During roasting, not only are new volatiles formed but also the loss of previously formed ones is observed, indicating the complex biochemical changes. Amongst the volatile compounds, furans appeared to be the most abundant ones.
Introduction
Turkish coffe is a world wide known traditional drink and is an important part of the daily life of Turkish people. The way it is prepared and served makes it different from the other cultures. 1 In 2013, it was listed as Intangible Cultural Heritage of Humanity by UNESCO.2 According to International Coffee Organization, 45 thousand tonnes of coffee was reported to be consumed in Turkey in 2016,3 which corresponded to 920 grams of coffee per person per year. In Turkey, coffee beans are roasted and brewed differently in different regions. In the southern provinces, coffee with darker colour, bitter taste and strong aroma is preferred and produced by roasting green coffee beans at higher temperature than ordinary Turkish coffee. Antakya coffee is one of this type as it is roasted at temperatures up to 235°C. Incidentally, unlike ordinary Turkish coffee which is served in small cup known as fincan, Antakya coffee is served in small tea glass known as süvari (cavalry in English).
It is well known that volatile compounds has a great impact on coffee flavor. To date, there have been more than 1000 volatiles identified in coffee.4 Roasting of green coffee beans directly affects the coffe aroma by degradation and formation or release of numerous chemical compounds through Maillard reactions, Strecker degradation, break down of amino acids, degradation of trigonelline, quinic acid, pigments, lipids and interaction between intermediate products.5
Up until now, limited studies have been conducted on ordinary Turkish coffee. So far, its antioxidant capacity, 6-7 furanic compounds, furfural content 8-9 and bioactive and mineral levels10 were revealed.11 determined the volatile and aroma profile together with descriptive analysis in boiled Turkish coffee. To the best of our knowledge, no study has been carried out to reveal the volatiles of Antakya coffee using high vacuum distillation technique.
Material and methods
Coffee samples
The coffee bean samples (Coffea arabica) supplied from a local coffee producer (Öksüzler Ltd. Co., Antakya). Bean samples were taken during the roasting at 140°, 200°, 230° and 235°C (corresponding to every 10 minutes of roasting), which were grounded by a coffee mill (BH259CG, BlueHouse, Turkey) before analysis.
High vacuum distillation
Fifty gram of grounded coffee samples were weighed in a Teflon bottle and extracted with 100 mL of diethly eter using a and a shaker (KS-15, Johanna Otto GmbH) for 20 min for each extraction. After each extraction, the extract were centrifuged at 4000 rpm (Rotanta 460, Hettich) for 5 min. This procedure was repeated three times. Supernatate was collected in a flask and frozen in liquid nitrogen before applying high vacuum at 10-5 torr in an apparatus described by.12 The high vacuum distillate was dried over sodiumsulphate, concentrated under nitrogen gas down to 1 mL before injecting to gas chromatography-mass spectrometry.
Gas chromatography-mass spectrometry analysis
The seperation of volatiles was carried out using a Hewlett-Packard 6890 GC/HP 5973 MS (Agilent Technologies) equipped with a fused silica capillary column INNOWax (60m in length, 0,2 mm id, 0,25µm film thickness). Hellium was the carrier gas. The GC-MS conditions were set as described by.12 Column gas flow rate was 1 mL min-1. Oven programme was 40° to 200 °C increase at a rate of 5°C min-1 with 5 and 45 min initial and holding time, respectively. MS conditions were as follows: interface temperature, 280°C; ionization energy, 70eV; mass rage, 30-330 amu; scaning rate, 2.2 scan sec-1. Injection volume was 1 µL in splittless mode. Volatile compounds were identified by comparing their mass spectra with the WILEY and NIST mass spectral (MS) libraries. Results were expressed as square root of each peak area. The experiment were repeated three times.
Results
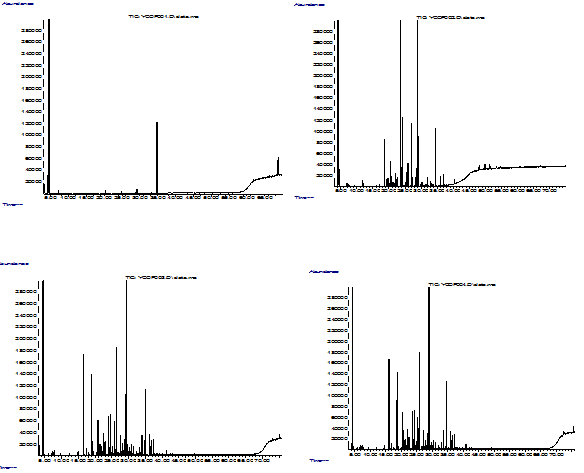
In this study, 36 volatile compounds were identifed in total. As seen from the chromatograms (Figure 1), the volatile profile of coffee beans continously changed as the roasting temperature increased, becoming more complex towards to end of roasting. In general, three stages were observed. In the first stage, volatile comopunds began to form when temperature exceeded 140°C and their amount and number increased continously up to 200°C. In the second stage, between 200° and 230°C, the fomation of new volatile compounds was observed while the amount some of the previous ones began to decrease. In the last stage, similar phenomenon to the second stage was observed, i.e. formation of new compounds and loss of previously formed ones, making the situation more complex.
|
Figure 1 Chromatograms showing the changes during roasting of Antakya coffee (a;140°C, b; 200°C, c; 230°C and d; 235°C). |
Volatile compounds identified were classified into three group as follows: (i) nitrogen containing heterocyclic compounds with 5 membered ring (pyrroles) or 6 membered ring (pyrazines, pyridines), (ii) oxygen containing heterocyclic compounds (furans), and (iii) miscellanous compounds (acids, ketons, esters, etc).
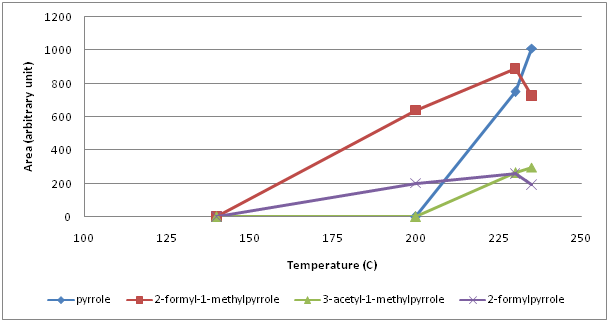
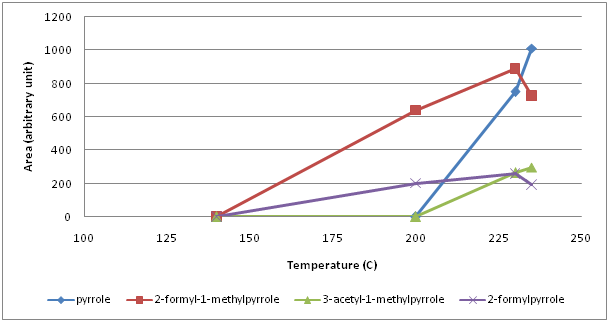
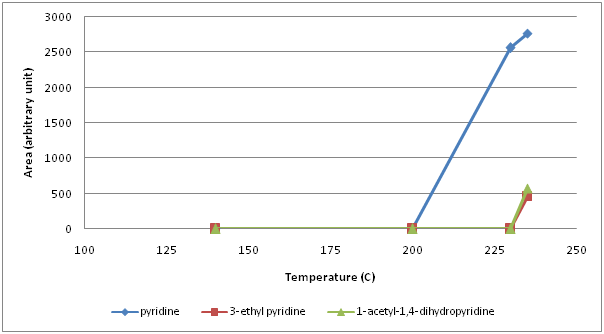
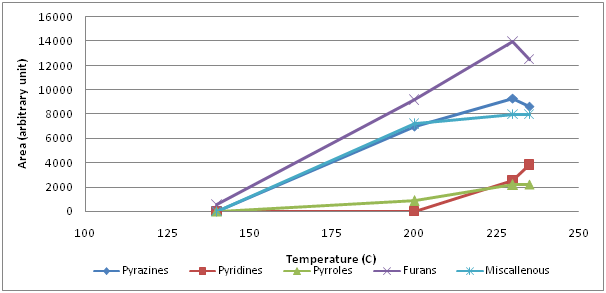
From the family of 5 membered hetreocyclic compounds containing 1 nitrogen, 5 pyrroles were identified (Figure 2). 2-formylpyrrole and 2-formyl-1-methylpyrrole were the first forming pyrroles between 140° and 200°C. When temperature exceeded 200°C, pyrrole and 3-acetyl-1-pyrrole formed and their amount continued to increase while 2-formylpyrrole and 2-formyl-1-methylpyrrole began to decrease. At 235°C, 1-furfurylpyrrole was detected. As 6 membered 2 nitrogen containing heterocylic compounds, 9 pyrazines (pyrazine, methyl pyrazine, 2,5-dimethyl pyrazine, 2,6-dimethyl pyrazine, 2-ethyl pyrazine, 2,3-dimethyl pyrazine, 2-ethyl-6-methyl pyrazine, 2-ethyl-3-methyl pyrazine, 2,3,5-trimethyl pyrazine) were identified (Figure 3). Amonst those, methyl pyrazine was the most abundant one. All pyrazines began to form within the first 10 min of roasting, that is between 140° and 200°C, except for pyrazine which formed above 200°C. Up to 230°C, the amount of the most pyrazines increased continuously thereafter a sharp decrease at 235°C was monitored. Unlike pyrazines, pyridines (6 membered heterocyclic compounds with 1 nitrogen) formed around 230°C (pyridine) or above 235°C (3-ethyl pyridine and 1-acetyl-1,4-dihydropyridine) (Figure 4).
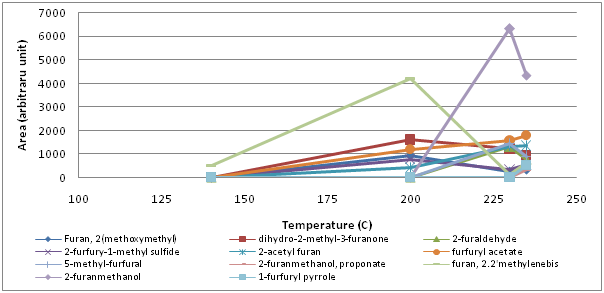
From the family of 5 membered hetreocyclic compounds containing oxygen, 10 furans were found (Figure 5). Between 140° and 200°C, 2-(methoxymethyl)furan, dihydro-2-methyl-3-furanone, 2-furfuryl-1-methylsulfide, 2-acetylfuran, furfurylacetate, 2-furfurylfuran were identified while 5-methyl-furfural, 2-furanmethyl propionate, 2-furanmethanol and 2-furaldehyde formed at higher temperatures (200° to 230°C). Amongst them, the behaviour of 2-furfurylfuran and 2-furanmethanol were notable. There was a sharp increase in 2-furanmethanol between 200°C and 230°C and a sharp decrease between 230°C and 235°C. Similar behaviour was observed for 2-furfurylfuran but at lower temperatures.
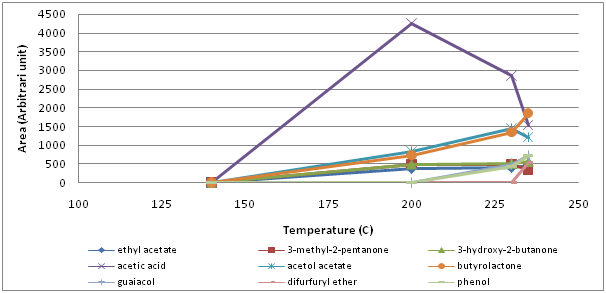
Finally, there were 9 miscellanous volatiles identified (ethyl acetate, 3-methyl-2-pentanone, 3-hydroxy-2-butanone, acetic acid, acetol acetate, butyrolactone, guaiacol, difurfuryl ether and phenol), in which acetic acid was the most abandunt one and showed the the biggest changes (Figure 6). Its amount increased rapidly and considerably up to 200°C and decreased thereafter.
In Figure 7, the overall changes were simplified arranging the total area of volatile compounds by their chemical class. It is clear that there was a steady increase in each chemical class up to 200°C. As the temperature exceeded 230°C, the amount of furans and pyrazines began to decrease owing possibly to thermal degradation while formation of pyrroles and pyridines tended to increase. Furans were the most abundant volatiles at all times followed by pyrazines and pyridines whereas pyrroles were the least formed ones.
Discussion and conclusion
It is well established that the cell structure ruptures during roasting of green coffee beans and moisture and the aromatic compounds that have been chemically bound are released from the beans. In parallel with this phenomena, starch in the beans converts sugar which later undergoes caramelization. Above 205°C pyrolysis starts giving rise to coffee flavour which deteriorates on severe roasting.13
Volatile compounds identified in this study have already been reported in several studies on different types coffee being mentioned in some reviews 5,14 as well as Turkish coffee.11 In agreement to the findings of, 11 furans were the major compounds in this study.
Several complex biochemical reactions take place during roasting. Furans forms via thermal degradation of carbohydrates, ascorbic acid, or unsaturated fatty acids.15,16 Furanones are generated in coffee mainly via Maillard reaction and subsequent aldol condensation.17 Nitrogen containing heterocyclic volatile compounds (pyrazine and pyrroles) forms via Strecker degredation which is a reaction between an amino acid and a-dicarbonyl with the formation of an aminoketone that condenses to form these compounds.5 Breakdown of hdyroxy-amino acids, viz serine and threonine, are able to react with a reducing sugar to form most alkylpyrazines. Pyridines also form decomposition of an alkaloid (trigonelline) that presents in coffe beans18 and by Maillard chemistry.19 Certain phenolic compounds like phenol, guaiacol arise from thermal degradation of chlorogenic acids (mainly ferulic, caffeic and quinic acids) and their concentration in roasted bean is proportional to the amount of chlorogenic acids present in green bean.20 Thermal degradation of pyroline and its reaction with intermediate Maillard products yield pyridines, pyrroles while thermal degradation of hdyroxypyroline and its reaction with Maillard products give rise to fufurylpyrroles.5 Pyrroles also forms degradation of trigonelline yields alkyl-pyrridines and pyrroles. 5
In conclusion, the formation of volatile compounds during roasting of Antakya coffee appears to be very much temperature dependent as expected, and the situation becomes very complex with increased roasting temperature. When 235°C was reached, the compounds for the dark colour and bitter taste and strong aroma of Antakya coffee are obtained. It is likely that this temperature has been set by the local producers by trial-and-error method traditionally and therefore the local producers avoid to exceed 235°C owing to the deterioration of flavour. This cultural know-how could be used for geographical labelling of Antakya coffee. It is evident that more research needs to be done on local products like Antaky coffee, which eventually could lead to regional development when correctly marketed.
References
- Aşık NA. Değişen kahve tüketim alışkanlıkları ve Türk kahvesi üzerine bir araştırma. Journal of Tourism and Gastronomy Studies. 2017;4:310‒325.
- Anonymous, 2013. https://ich.unesco.org (visited on 05.02.2018).
- Anonymous, 2016. www.ico.org (visited on 05.02.2018)
- Nijssen LM, Visscher CA, Maarse H, et al. Volatile compounds in food: qualitative and quantitative data. Central Institute for Nutrition and Food Research, TNO: Zeist, The Netherlands. 1996.
- Buffo RA, Cardelli‐Freire C. Coffee flavour: an overview. Flavour and Fragrance Journal. 2004;19(2):99‒104.
- Karakaya S, El SN, Taş AA. Antioxidant activity of some foods containing phenolic compounds. Int J Food Sci Nutr. 2001;52:501–508.
- Gündüç N, El SN. Assessing antioxidant activities of phenolic compounds of common Turkish food and drinks on in vitro low-density lipoprotein oxidation. Journal of Food Science. 2003;68:2591–2595.
- Chaichi M, Ghasemzadeh-Mohammadi V, Hashemi M, et al. Furanic compounds and furfural in different coffee products by headspace liquid-phase micro-extraction followed by gas chromatography–mass spectrometry: Survey and Effect of Brewing Procedures. Food Additives and Contaminants Part B. 2015;8(1):73–80.
- La Pera L, Liberatore A, Avellone G, et al. Analysis of furan in coffee of different provenance by head-space solid phase microextraction gas chromatography–mass spectrometry: Effect of Brewing Procedures. Food Additives and Contaminants Part A. 2009;26:786–792.
- Özdestan Ö. Evaluation of bioactive amine and mineral levels in Turkish coffee. Food Research İnternational. 2014;61:167‒175.
- Sarrazin C, Le Quéré JL, Gretsch C, et al. Representativeness of coffee aroma extracts: a comparison of different extraction methods. Food Chemistry. 2000;70(1):99‒106.
- Avsar YK, Karagul-Yuceer Y, Drake MA, et al. Characterization of nutty flavor in Cheddar cheese. J Dairy Sci. 2004;87(7):1999‒2010.
- Bhumiratana N, Adhikari K, Chambers IV E. Evolution of sensory aroma attributes from coffee beans to brewed coffee. LWT-Food Science and Technology. 2011;44(10):2185‒2192.
- Sunarharum WB, Williams DJ, Smyth HE. Complexity of coffee flavor: A compositional and sensory perspective. Food Research International. 2014;62:315‒325.
- Crews C, Castle L. A review of the occurrence, formation and analysis of furan in heat-processed foods. Trends in Food Science and Technology. 2007;18(7):365‒372.
- Ribeiro JS, Augusto F, Salva TJ, et al. Prediction of sensory properties of Brazilian Arabica roasted coffees by headspace solid phase microextraction-gas chromatography and partial least squares. Anal Chim Acta. 2009;634(2):172‒179.
- Grosch W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem Senses. 2001;26(5):533‒545.
- Zheng X-Q, Ashihara H. Distribution, biosynthesis and function of purine and pyridine alkaloids in Coffea arabica seedlings. Plant Science. 2004;166(3):807–813.
- Mottram D. The maillard reaction: Source of flavour in thermally processed foods. In: Berger R., editor. Flavours and fragrances. Springer; Berlin Heidelberg: 2007. pp. 269–283.
- Bicho NC, Leitão AE, Ramalho JC, et al. Impact of roasting time on the sensory profile of Arabica and Robusta coffee. Ecology of Food and Nutrition. 2013;52(2):163‒177.